BluePrint Orphan
@BluePrintOrphan
Proven excellence in #orphandrug data analytics, forecasting, and market access strategy with a special focus in #RareDiseases *retweets/likes ≠ endorsements*
You might like
Have you been wondering how #COVID19 will affect #orphandrug development? Check out our latest blog post about the pandemic’s impact on the #raredisease sector blueprintorphan.com/development/co…
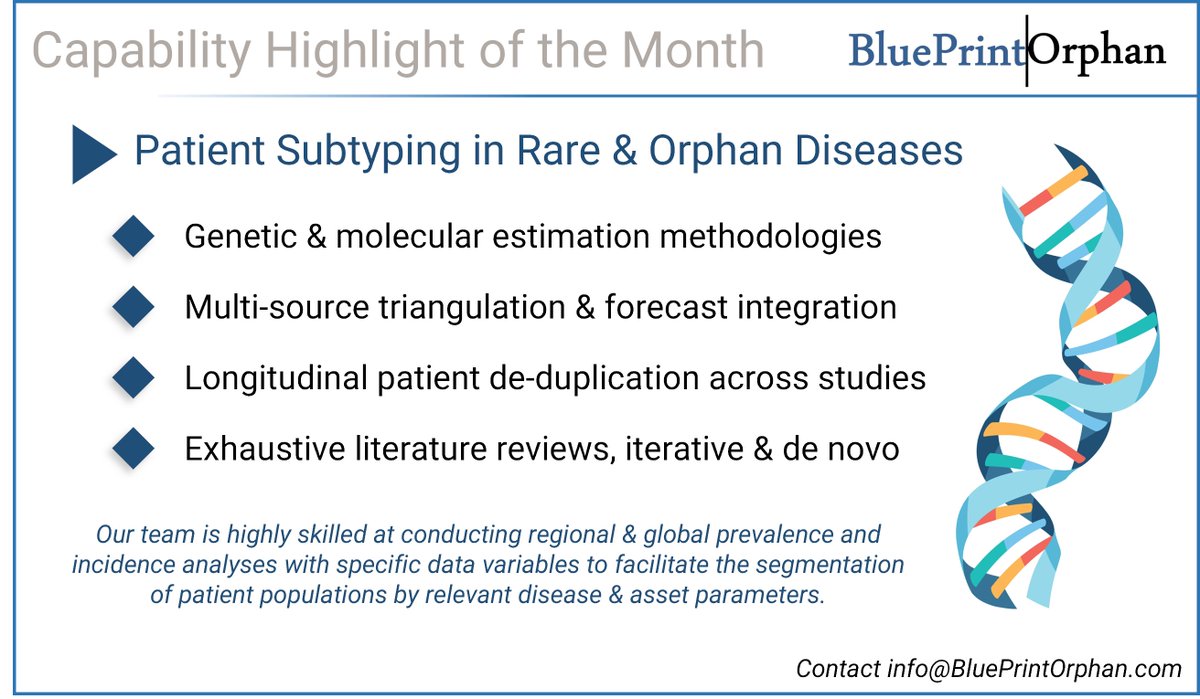
Highlighting our Patient Subtyping capabilities this month. Want to know more? Contact our team today! #raredisease #orphandrug
Curious about #raredisease treatment development progress in 2020? Check out some of this year's drug approvals! pharmaceutical-technology.com/features/treat…
Great to see everyone on screen at day 1 of Inborn Errors of Metabolism today! Looking forward to more discussion tomorrow #RareDiseases inborn-errors.com
Interesting read on how machine learning can be used to help fight #rarediseases pharmalive.com/machine-learni…
The #NORDSummit will bring #raredisease leaders & stakeholders together to provide new approaches & fresh perspectives on #publicpolicy, clinical trials, access to #diagnostic testing & pharmaceutical innovation. Register or apply for a scholarship: bit.ly/2WJ7ziE

Interesting article highlighting the inherent challenges of #orphandrug research and development - our team is here to help! euractiv.com/section/health…
euractiv.com
The complexity of R&D for orphan medicines | Euractiv
There are multiple challenges inherent to drug development for rare diseases, all consequences of the small number of patients. For most of these diseases, the body of pre-existing knowledge is...
Join our experts for a free webinar: The Value of Orphan Drug Designation in your Drug Development Program ow.ly/9KBx50AmOra #OrphanDrug #RareDisease #ClinicalDevelopment #Regulatory #FreeWebinar #ClinialTrials #ClinicalStudies #Innovation Cato Research

With the #DISORDER: The #RareDisease Film Festival postponed by the pandemic, the Rare Outreach Coalition decided to start its own streaming channel and bring their content right into your home! Read more: bwnews.pr/2Obbpww
Thanks to these four families and @nytimes for profiling what life with #raredisease is really like - #orphandrugs and treatments are so important nytimes.com/2020/07/07/hea…
Congrats to #IsaPharmaceuticals for receiving @US_FDA #OrphanDrug Designation for ISA101b in HPV16-positive cervical cancer prnewswire.com/news-releases/…
prnewswire.com
ISA Pharmaceuticals Receives US Orphan-Drug Designation for ISA101b in HPV16-positive Cervical...
/PRNewswire/ -- ISA Pharmaceuticals B.V., a clinical-stage company dedicated to developing rationally designed immunotherapeutics for oncology and infectious...
LAST CALL to register for #RareAcrossAmerica Check out this article from @healourskin where Shannon von Felden, #RDLA program director, shares everything you need to know about this unique event. issuu.com/healours…/docs/ippf-100-spring-20-issuu/26
Interesting article on the discovery of novel genetic causes of #rarediseases healtheuropa.eu/genome-sequenc…
Join #NORD, @CPathInstitute, @US_FDA and @TakedaPharma tomorrow, June 24 at 2:00 PM ET for “Shortening the Timeline for Developing New Treatments – How the Rare Disease Cures Accelerator–Data and Analytics Platform Can Help.” Register: bit.ly/2Bxd10k #RDCADAP #webinar

Excited to see new funding opportunities for #raredisease research 🎉
The National Organization for Rare Disorders (NORD) is pleased to announce new funding opportunities through the Jayne Holtzer #RareDisease #Research Grants Program. Submission of proposals are due by August 25. Read more: bit.ly/2ANmLDO
Congrats to #ProtagonistTherapeutics on receiving #OrphanDrug Designation for hepcidin mimetic PTG-300
The National Organization for Rare Disorders (NORD) is pleased to announce new funding opportunities through the Jayne Holtzer #RareDisease #Research Grants Program. Submission of proposals are due by August 25. Read more: bit.ly/2ANmLDO
The @US_FDA has announced that #raredisease patients and #orphandrugs remain priorities during #COVID19 fda.gov/news-events/fd…
Check out this fascinating development in #raredisease diagnostic technology for childhood genetic conditions sciencetimes.com/articles/25950…
Thanks to Dr. @DavidFajgenbaum for sharing your perspective on how #raredisease and #orphandrug research may apply to #COVID19 phillyvoice.com/covid-19-treat…
Today until July 31, the @US_FDA is seeking comments and feedback from patients, health professionals, and others in the #raredisease community to establish a clinical trials network federalregister.gov/documents/2020…
Interesting read on how #orphandrug biotech @Pharnext plans to respond to #COVID19 by leveraging AI data forbes.com/sites/lawrence…
United States Trends
- 1. #TerrorismProvocation N/A
- 2. Good Tuesday N/A
- 3. FINALLY DID IT N/A
- 4. Davos N/A
- 5. #tuesdayvibe N/A
- 6. Macron N/A
- 7. The Jito N/A
- 8. #CatForCashEP1 N/A
- 9. Deloitte N/A
- 10. The ONDO N/A
- 11. Taco Tuesday N/A
- 12. #WWEUnreal N/A
- 13. Memories in Orbit N/A
- 14. #KidnappedPresident N/A
- 15. Weston Wilson N/A
- 16. Phillips N/A
- 17. Diego Garcia N/A
- 18. Saleh N/A
- 19. Scott Jennings N/A
- 20. Alberto N/A
You might like
Something went wrong.
Something went wrong.