FDAAA TrialsTracker
@FDAAAtracker
Which trials have not reported their results according to the FDAAA? Find out on the FDAAA TrialsTracker! A project from @BennettOxford
You might like
Just a heads up, the FDAAA and EU TrialsTracker will be seeing some potential downtime over the next few weeks as we do some rearranging internally.
We are launching a project on improving code sharing in health and medical research funded by @UKRI_News. Read more here: bennett.ox.ac.uk/blog/2025/08/h…
You may have noticed we haven't updated the TrialsTracker in a while due to some technical issues. Well good news! Those have been fixed now and you can expect regular updates to resume moving forward! fdaaa.trialstracker.net
WHO highlighting best practice for transparency of trials aligned with revised Dec of Helsinki. Trialists should: -Prospectively register -Report within 12 months of completion -Share results with participants -Plan to responsibly share trial data linkedin.com/posts/vasee-mo…
Just a note, the fdaaa.trialstracker.net had not been updated in some time as we needed to make some updates to our software to account for the new ClinicalTrials.gov website. Those have now been made at the data is once again current!
WE ARE HIRING! A prestigious fellowship at @JesusOxford working with us on your own projects around epidemiology, open science, research software engineering and all our usual obsessions! @BennettOxford @OpenSAFELY @openprescribing @FDAAAtracker and more bennett.ox.ac.uk/blog/2023/02/w…
WE ARE HIRING Junior Research Fellow with college affiliation. That means meals and rich social/intellectual community on top of 3 years with the mighty team that builds @OpenSAFELY @FDAAAtracker @openprescribing Come change the world through data and tools!
APPLY NOW!! With @JesusOxford we're seeking a Bennett Institute Junior Research Fellowship. 3 years to come and work with our fantastic team, with all the benefits of college affiliation. jesus.ox.ac.uk/about-jesus-co…
Come and work with us. This is an unprecedentedly awesome job. Three years with the team that builds @OpenSAFELY @FDAAAtracker @openprescribing AND Oxford college affiliation, with all the dinners and discursive chats across disciplines that entails!
APPLY NOW!! With @JesusOxford we're seeking a Bennett Institute Junior Research Fellowship. 3 years to come and work with our fantastic team, with all the benefits of college affiliation. jesus.ox.ac.uk/about-jesus-co…
Wrote up some quick thoughts about using the new EU CTIS registry: bennett.ox.ac.uk/blog/2022/10/f…
ICYMI, I had two pieces on pre-registration come out last week with a bunch of wonderful co-authors. Primer on Registration in @BMJ_EBM: ebm.bmj.com/content/early/… Editorial on UK Registration in @bmj_latest: bmj.com/content/376/bm…
In news probably only a handful of people care about, but drove me crazy, ClinicalTrials.gov is finally closing a loophole that let people submit delays of results for good cause much later than should be allowed under FDAAA @reshmagar @TranspariMED @DeborahZarin

Consistent with our findings from the @FDAAAtracker on sponsor size being a major predictor of FDAAA compliance Here: thelancet.com/journals/lance… And here: jamanetwork.com/journals/jamai… And consistent with the EU as well here: bmj.com/content/362/bm…
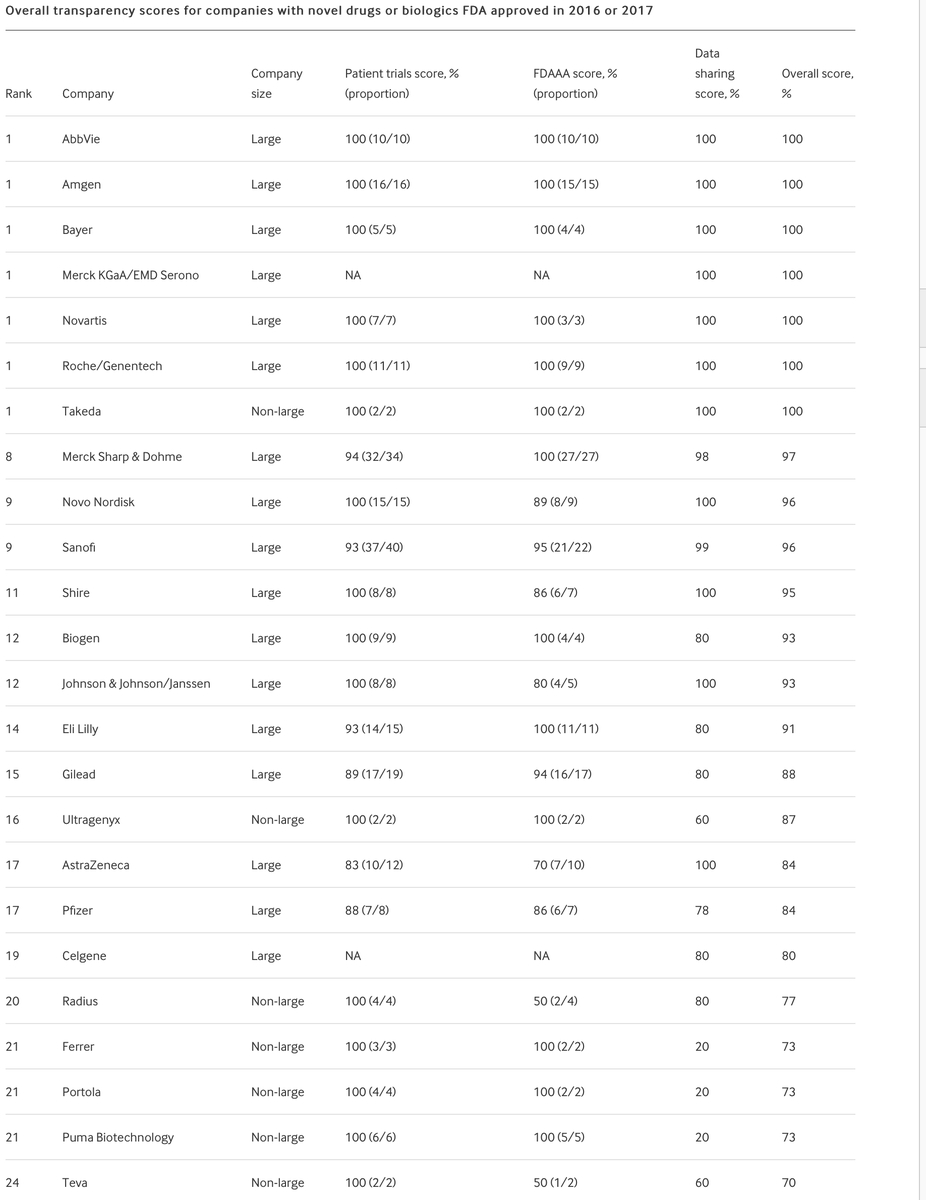
Our paper ranking 42 pharma Cos on research transparency & data sharing is out. We found large Cos are more transparent than non-large, driven by better data sharing & FDAAA implementation @syd_axson @MichelleM_Mello @cpgYALE @jsross119 C.Yang @BMJ_Open bmjopen.bmj.com/content/11/7/e…
My group in Oxford has published LOTS on who does, and doesn't, report their clinical trial results. But what about the rest: late registrations, data verification, certificates of delay, document sharing? Our @NDevito1 lashes detail in @JAMAInternalMed jamanetwork.com/journals/jamai…
New from me and @bengoldacre in @JAMAInternalMed: We expanded our earlier work in @TheLancet examining results reporting requirements of the FDA Amendments Act 2007 to additional areas of compliance across the entire dataset. jamanetwork.com/journals/jamai…
I'll shout more about this when it isn't Friday evening but my preprint on COVID trial reporting with @PeterRolandG and @maiameta @Strech_Da from @questbih is up! Check it out for a weekend read!
Results Availability and Timeliness of Registered COVID-19 Clinical Trials: A Cross-Sectional Study medrxiv.org/cgi/content/sh… #medRxiv
The @US_FDA are under fire for (again) failing to monitor and enforce on non-compliance with trial reporting rules. Our @FDAAAtracker (in the piece) does the monitoring job fine, and didn't cost much to build: they could use that, or build their own. statnews.com/pharmalot/2020…
Pleased to share something I've been working on for the past couple weeks. The newest addition to our TrialsTracker.net project: covid19.trialstracker.net
Just won (mostly) a court case against @us_fda: agency can no longer exempt certain trial results from reporting on clinicaltrials.gov contrary to law (FDAAA). Thx to co-plaintiff @DrPeterLurie & litigators @MFIAclinic @nyulaw @Yale_CRIT. documentcloud.org/documents/6784…
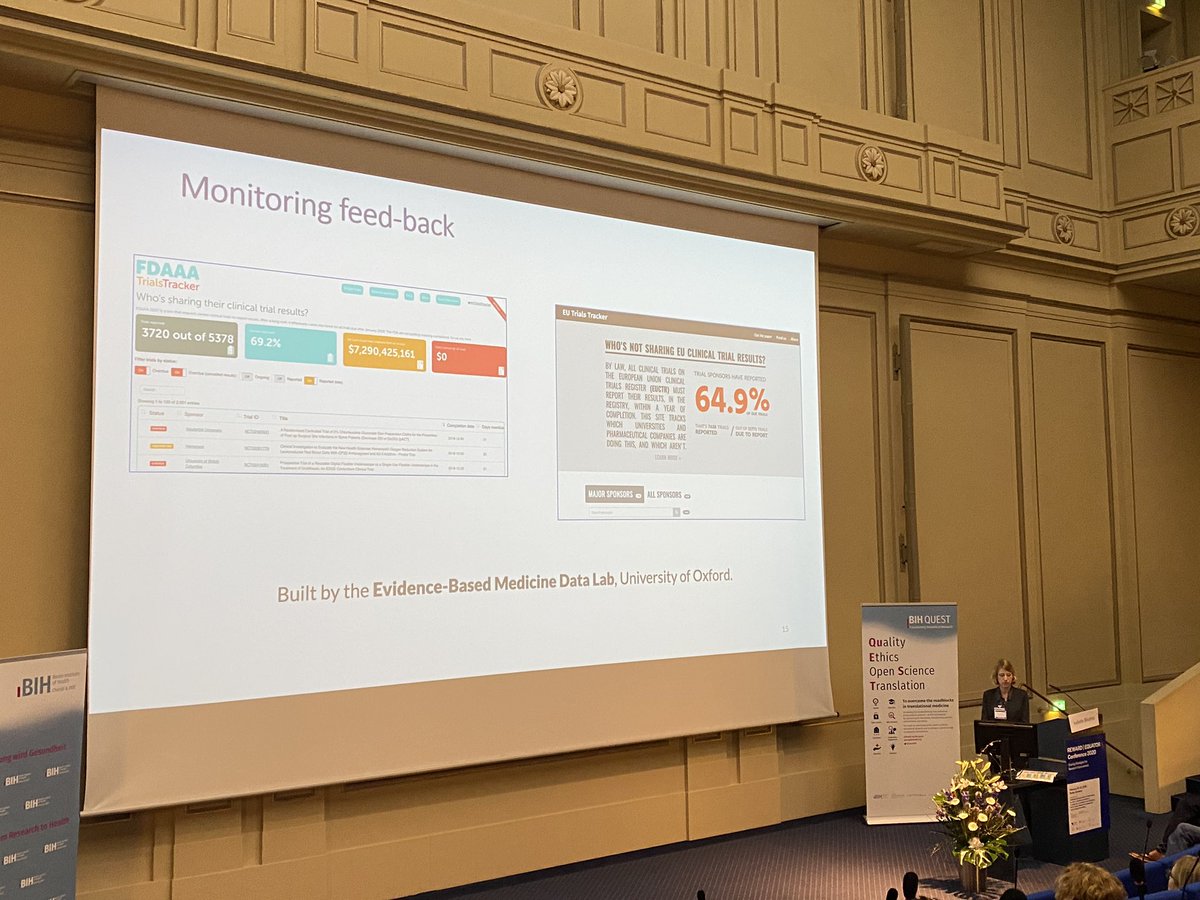
In the Doug Altman Lecture at #REWARD2020 @BOUTRON1 talks a about our TrialsTracker work.
Our paper!
Musings re the boring-sounding technical problem of "publication bias", in which unsuccessful drug trials aren't published, and why the real-world upshot is that people die because they're prescribed drugs that don't work. WRT @bengoldacre's latest study unherd.com/2020/01/why-th…
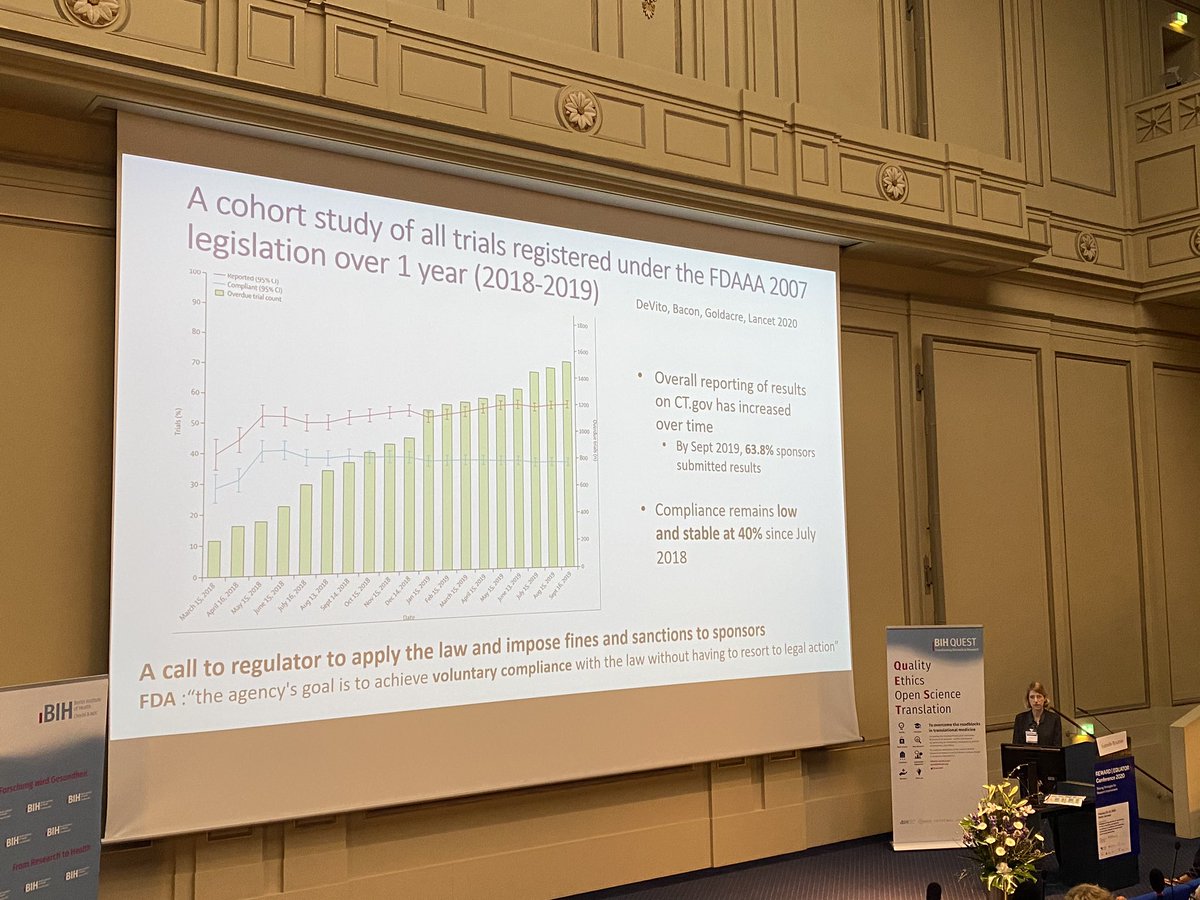
BING BONG NEW PAPER @NDevito1 @sebbacon and me in the LANCET today. We definitively assess compliance with US law FDAAA 2007, which requires clinical trials to report results within 12 months of completion. The answer: 59% of trials breached the law thelancet.com/journals/lance…
United States Trends
- 1. Good Thursday N/A
- 2. #thursdayvibes N/A
- 3. #AgendaEnergética N/A
- 4. Happy Friday Eve N/A
- 5. #DareYouToDeathEP8 N/A
- 6. LINGORM EMDISTRICT CNY2026 N/A
- 7. #EMDISTRICTCNY2026xLingOrm N/A
- 8. JUNGKOOK N/A
- 9. Jessie Diggins N/A
- 10. #ThursdayThoughts N/A
- 11. Timo N/A
- 12. Día de la Juventud N/A
- 13. Richard Gere N/A
- 14. ICE Custody N/A
- 15. Scott Jennings N/A
- 16. Leon Black N/A
- 17. Latvia N/A
- 18. The IOC N/A
- 19. Vote YES N/A
- 20. $NBIS N/A
You might like
-
 aussie17
aussie17
@_aussie17 -
 André Martin Mansoor
André Martin Mansoor
@AndreMansoor -
 @samizdathealth
@samizdathealth
@DrDavidHealy -
 Danielle Ofri
Danielle Ofri
@danielleofri -
 Less-Is-More Cardiologist
Less-Is-More Cardiologist
@DavidLBrownMD -
 Rohan S.
Rohan S.
@RSiddhanti -
 Matthew Watto MD FACP
Matthew Watto MD FACP
@DoctorWatto -
 METRICStanford
METRICStanford
@METRICStanford -
 Todd C. Lee
Todd C. Lee
@DrToddLee -
 Emirates Society of Ophthalmology
Emirates Society of Ophthalmology
@ESO_UAE -
 Joseph Ross
Joseph Ross
@jsross119 -
 Chris Hendel
Chris Hendel
@chrishendel
Something went wrong.
Something went wrong.