MDDAProgram
@MddaProgram
The Medical Device Discovery Appraisal Program
You might like
The latest @MDIConline @caseforquality Case for Quality Forum sessions are posted now: mdic.org/resource/case-… with updates from @CMMI_Institute and @FDADeviceInfo Let us know if you have any questions on @MddaProgram or the Case for Quality
Have questions about our Medical Device Discovery Appraisal Program? Check out our FAQs page to learn more about program basics, the appraisal process, and how to enroll. bit.ly/3aHrJOQ
A bit on the Case for Quality Voluntary Improvement Program at 3:30 into this podcast. The program is growing with close to a 100 appraisals performed to date. Contact us if you are interested or have questions @CMMI_Institute @MDIConline overcast.fm/+DjJi-CJTo
overcast.fm
Overcast
Device Week, 13 March 2020 – Paying For COVID-19 Tests, MDIC’s Case...
From the tail of last year, but insightful related to the direction for some of the elements of the Case for Quality. youtube.com/watch?v=pIvLWQ…
youtube.com
YouTube
THE FUTURE OF QUALITY MANAGEMENT AND FDA CASE FOR QUALITY INITIATIVES
Join MDIC for our next Case for Quality forum, co-hosted with @MITREcorp, in McLean, VA on March 12, 2020. We'll provide an update on the status of the VIP Operational Program and planned CfQ activities. Learn more and register: bit.ly/39IyqiV

Curious about the Medical Device Discovery Appraisal Program (MDDAP)? A program for medical device stakeholders to work together to enhance device quality and patient safety by leveraging the #CMMI model. Learn more on our website today. bit.ly/2pdQaxx
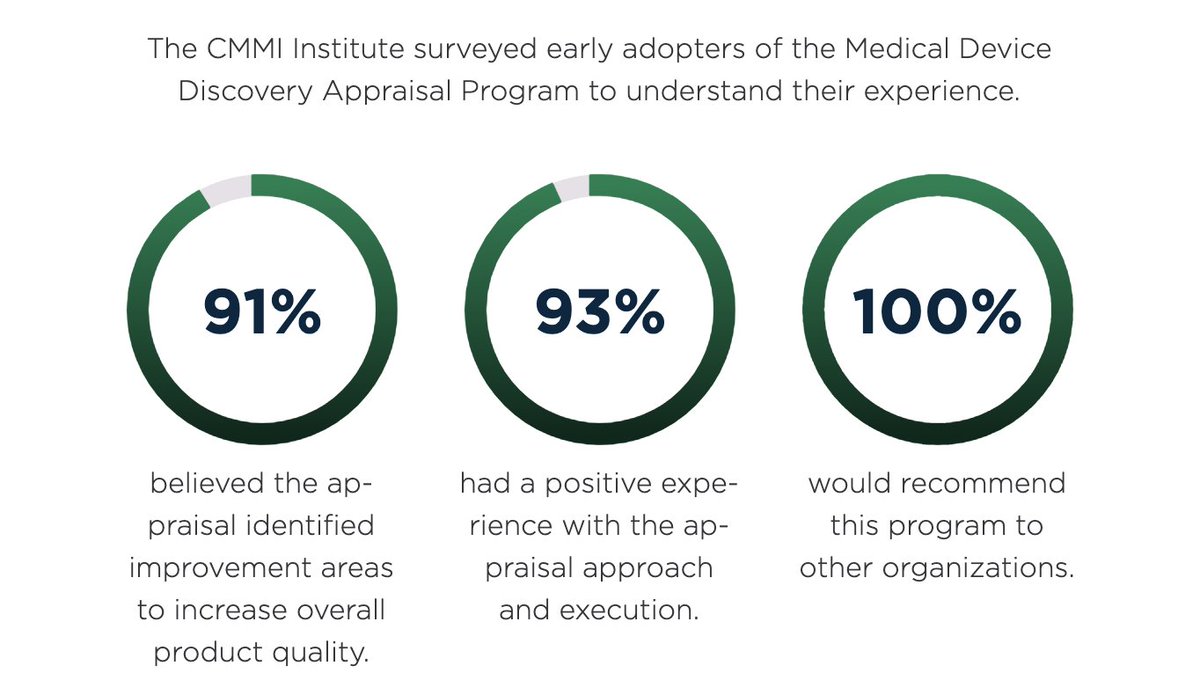
The @CMMI_Institute has published the @FDADeviceInfo written white paper that describes the state of the @MddaProgram pilot program through September of 2019. Read / download it here: cmmiinstitute.com/getattachment/… @caseforquality
Woot! @CMMI_Institute Model at a glance reference material. Contact us if interested the model and improvement!

JUST RELEASED: Our Case for Quality program offers a new whitepaper recasting CAPA as a continuous improvement process. Learn more: bit.ly/2LleyZs

The latest Greenlight Guru podcast on the Case For Quality Voluntary Program. Contact us if interested or if you have questions. overcast.fm/+FIAGB2pzQ
medtech.pharmaintelligence.informa.com/MT125860/FDAs-… - not for “naughty” firms but possibly for groups looking to improve.
Don't miss our final Case for Quality Forum of 2019! Learn more and register here: bit.ly/2mplVWC

FDA Releases Case for Quality Pilot Program Report, Seeks Feedback - #MDIC #CMMI #MDDAP bit.ly/2oHl5p3
1/2 “That's why the FDA is collaborating with the MDIC and Pittsburgh, PA-based CMMI Institute to develop a variation of the VIP program for struggling sites” — read more in the latest @Medtech_Insight article about the collaboration between @FDADeviceInfo, ...
Did you know MDIC hosted 12 MDICx webinars in 2018? That's just one of the many highlights from our 2018 Annual Report. Read the full report here: bit.ly/2XAAd42
REGISTRATION OPEN: Join us for MDIC's 2019 Annual Public Forum on September 5 in Washington, DC. Follow @MDICAnnualForum for the latest updates. Register here: bit.ly/2I4117z

With the success of the #MedicalDevice Discovery #Appraisal Program (#MDDAP) pilot, @MDIConline is working with @US_FDA & #CMMI Institute to formalize a full program, now known as the #CaseforQuality. Attend this #MDICx #webinar tomorrow to learn more: bit.ly/2W6uJQY

Join us on June 20 for our Case for Quality Forum in Arlington, VA, co-hosted by @CMMI_institute. Register by June 18. bit.ly/2YtYqsJ @FDADeviceInfo

Check in as to what is occurring with #MDDAP
Join us for a MDICx this Wednesday for an update on the Case for Quality Voluntary Improvement Program (CfQ VIP). For more details or to register visit: bit.ly/2GGiF0y

The FDA hosts their annual Regulatory Education for Industry session May 29-30. It is FREE! And you can attend virtually (in fact, that is the only way you can attend now!) You can get more information on it here: sbiaevents.com/redi2019/?utm_…
United States Trends
- 1. Happy Thanksgiving Eve 1,249 posts
- 2. Good Wednesday 21.2K posts
- 3. Luka 65K posts
- 4. #DWTS 97.3K posts
- 5. Lakers 50.8K posts
- 6. Clippers 18.9K posts
- 7. Robert 140K posts
- 8. Kris Dunn 2,874 posts
- 9. Collar 46K posts
- 10. Jim Mora 1,022 posts
- 11. #LakeShow 3,590 posts
- 12. Kawhi 6,511 posts
- 13. Karoline Leavitt 24.7K posts
- 14. Reaves 13.5K posts
- 15. Alix 15.2K posts
- 16. Jaxson Hayes 2,626 posts
- 17. Colorado State 2,610 posts
- 18. TOP CALL 14.6K posts
- 19. Elaine 46.5K posts
- 20. Ty Lue 1,662 posts
Something went wrong.
Something went wrong.