Neurotech International
@NeurotechInt
Neurotech is a clinical-stage biopharmaceutical development company focused on paediatric neurological disorders.
You might like
Our Quarterly Activities Report for the period ending 30 September 2025 has been released. View the full report here: 🔽 api.investi.com.au/api/announceme… $NTI #Neurotech #Biotech #RettSyndrome #Autism #PANDAS #PANS

In the latest episode of @StockheadAU's Who’s Who on the ASX, host Tylah Tully highlights #NTI164, our broad-spectrum oral cannabinoid therapy in development for paediatric neurological disorders. Read the full $NTI article here: stockhead.com.au/stockhead-tv/w…
2025 Annual Report View the full Annual Report here: api.investi.com.au/api/announceme… $NTI #Neurotech #Biotech #RettSyndrome #Autism #PANDAS #PANS

📰Neurotech Secures Rare Pediatric Disease Designation for NTI164 in Rett Syndrome | $NTI Media coverage by @techinvestmag. techinvest.online/news/neurotech… #NTI #Neurotech #Biotech #RettSyndrome

October 9 is PANS/PANDAS Awareness Day, a time to recognise the sudden and challenging nature of these neuroimmune disorders. At Neurotech, we’re advancing NTI164 with a focus on immune regulation and gene expression; two biological pathways central to what drives PANDAS/PANS.…

Neurotech scores US FDA rare disease win for NTI164 in Rett syndrome | $NTI Media coverage by @australian theaustralian.com.au/business/stock… #Neurotech #RettSyndrome #Biotech #ASX #ASXNews

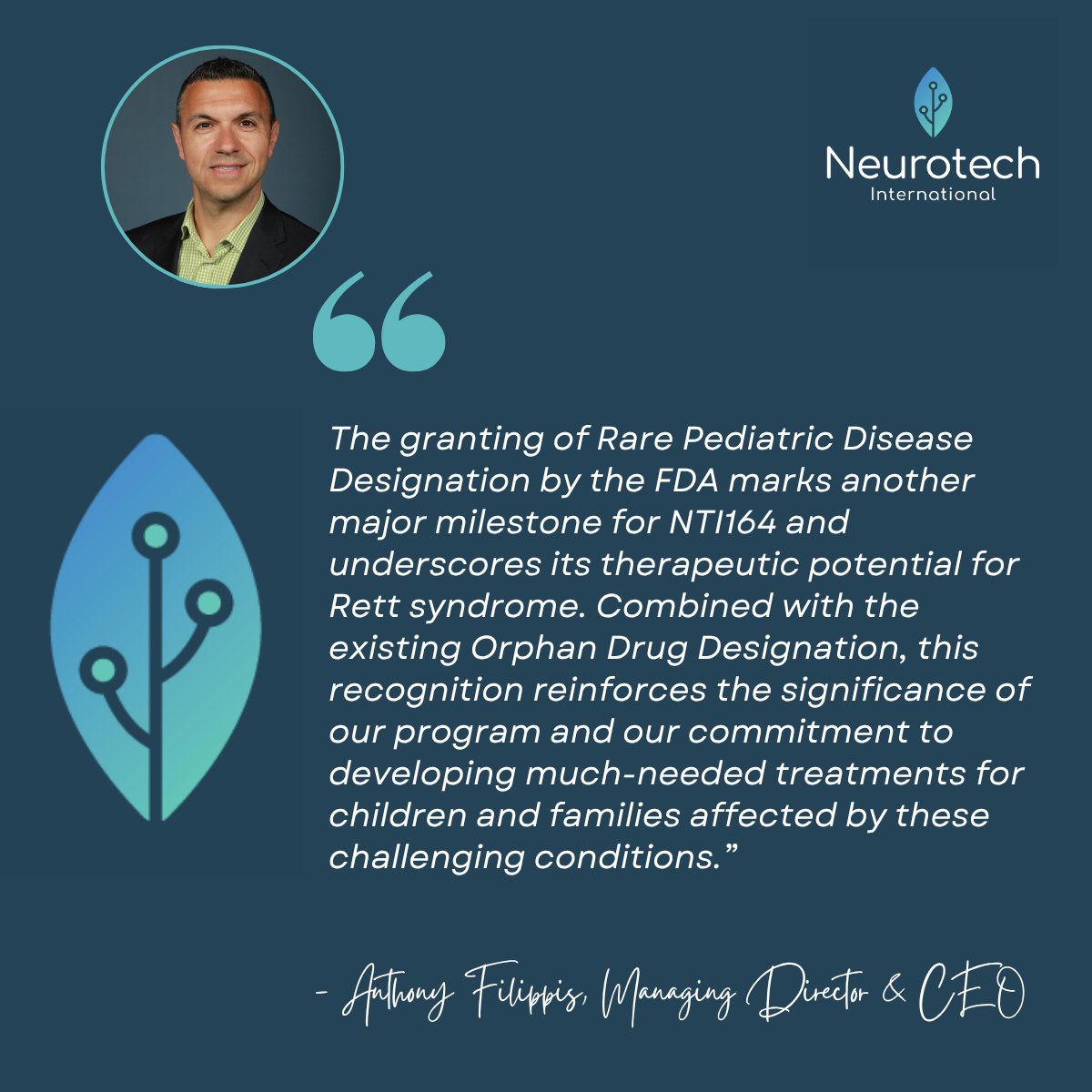
We’ve received Rare Pediatric Disease Designation from the US FDA for NTI164 in Rett syndrome. This milestone recognition complements the existing Orphan Drug Designation and strengthens our position in developing therapies for rare and underserved neurological disorders. The…
CEO Anthony Filippis outlines how Neurotech is advancing NTI164 through clinical development in the latest article featured in Stockhead. Anthony discusses the initiation of the Authorised Prescriber program and highlights our focus on accelerating regulatory pathways, creating…

October marks Rett Syndrome Awareness Month, a time to recognise the challenges faced by children and families living with this rare neurological disorder. Rett Syndrome affects approximately 1 in 10,000-15,000 girls, and currently, there is no cure. It is caused by mutations in…

$NTI coverage in the @australian via @StockheadAU – "Neurotech launches Authorised Prescriber program for its neurodevelopmental disorders therapy". Click here to read: theaustralian.com.au/business/stock…

We are pleased to announce the commencement of an Authorised Prescriber (AP) program for our therapy NTI164, across a range of neurodevelopmental conditions for paediatric patients in Australia: api.investi.com.au/api/announceme… The program will be managed by leading paediatric…

Our Principal Investigator, Professor Russell Dale, who leads our Phase I/II PANDAS/PANS clinical trial, has had a new paper published in @Nature (Molecular Psychiatry). This recognition underscores the calibre of clinical leadership guiding NTI164’s development and builds…

“We now have the clinical data, regulatory momentum, and leadership to deliver NTI164 to patients globally,” Chairman Mark Davies said. FY25 was a turning point,” Davies said. “With a strong clinical package, regulatory recognition, and an experienced team, Neurotech is…

"In my clinical practice, I’ve seen how full-spectrum cannabinoid therapies can profoundly improve the lives of children with autism, Rett syndrome, and PANS." – Dr. Bonni Goldstein MD, Neurotech Chief Medical Advisor (USA). Dr. Goldstein supports NTI164, Neurotech’s consistent,…
The U.S. is considering moving #marijuana from a Schedule I to a Schedule III substance, a change that could make life a lot easier for companies working with #cannabinoid-based medicines. While #NTI164 is already developed and trialled within Australia’s regulatory framework,…

Our Managing Director & CEO, Anthony Filippis joined today’s “Deal Making: The Real Story” panel at the 2025 Bioshares Biotech Summit, alongside fellow biotech leaders sharing real-world lessons on navigating transactions, partnerships, and growth. Thanks to Bioshares for…

We’ve been featured in @TheAustralian in an article exploring new treatments for Rett syndrome, a rare and complex paediatric neurological condition. Read more: theaustralian.com.au/business/stock… $NTI #Neurotech #ASX #NTI #RettSyndrome #NTI164 #PaediatricNeurology #CannabinoidTherapy…

"Neurotech’s June quarter was marked by important clinical progress, growing international recognition, and further regulatory engagement for its lead investigational therapy #NTI164, a proprietary #CBDA-rich #cannabinoid formulation being developed for #paediatric neurological…

United States Trends
- 1. #SmackDown 33K posts
- 2. Caleb Wilson 3,972 posts
- 3. Giulia 11.1K posts
- 4. #OPLive 1,135 posts
- 5. Lash Legend 3,952 posts
- 6. #TheLastDriveIn 1,618 posts
- 7. Chelsea Green 4,765 posts
- 8. Reed 24.8K posts
- 9. #BostonBlue 1,418 posts
- 10. Darryn Peterson 2,023 posts
- 11. Kansas 22.8K posts
- 12. Supreme Court 158K posts
- 13. Sengun 3,675 posts
- 14. Rockets 17.8K posts
- 15. #Dateline N/A
- 16. Harrison Barnes N/A
- 17. End of 3rd 1,380 posts
- 18. Nia Jax 2,628 posts
- 19. End 3Q N/A
- 20. Dizzy 12.4K posts
You might like
-
 Brightstar Resources
Brightstar Resources
@BrightstarReso1 -
 Lincoln Minerals
Lincoln Minerals
@LincolnMinerals -
 Dynamic Metals (ASX:DYM)
Dynamic Metals (ASX:DYM)
@DynamicMet_DYM -
 Ushuru Sacco Limited™
Ushuru Sacco Limited™
@UshuruSacco -
 OD6 Metals
OD6 Metals
@Od6Metals -
 HyTerra
HyTerra
@HyTerra_ASX -
 Iceni Gold
Iceni Gold
@Iceni_Gold -
 Ballymore Resources
Ballymore Resources
@BallymoreRes -
 GoldenDeepsLtd
GoldenDeepsLtd
@GoldenDeepsLtd -
 ALAIN BRO
ALAIN BRO
@alain_bro -
 islandpharmaceuticals
islandpharmaceuticals
@IslandPharma -
 Equinox
Equinox
@Equinox_EQN
Something went wrong.
Something went wrong.