Scendea
@scendea
Scendea is a leading product development and regulatory consulting practice serving the pharmaceutical and biotechnology industry.
你可能會喜歡
Last week, Scendea had the pleasure of attending The UniSC's 10-year Anniversary Clinical Trials Conference in QLD, Australia. - Book a meeting with our award-winning team, and discover how we can support your #clinicaltrials in Australia: scendea.com/contact

Read and download our latest Whitepaper, "Reference Product Sourcing and Regulatory Planning for Biotech Products", written in collaboration with Acnos Pharma - scendea.com/articles/refer… - Read and download the paper, today: - #whitepaper #drugdevelopment




The @US_FDA has announced a pilot program designed to streamline communication with sponsors following formal meetings. Discover how Scendea can support your FDA interactions, book a meeting with our team, today: scendea.com/contact - #FDAinteractionsupport #drugdevelopment




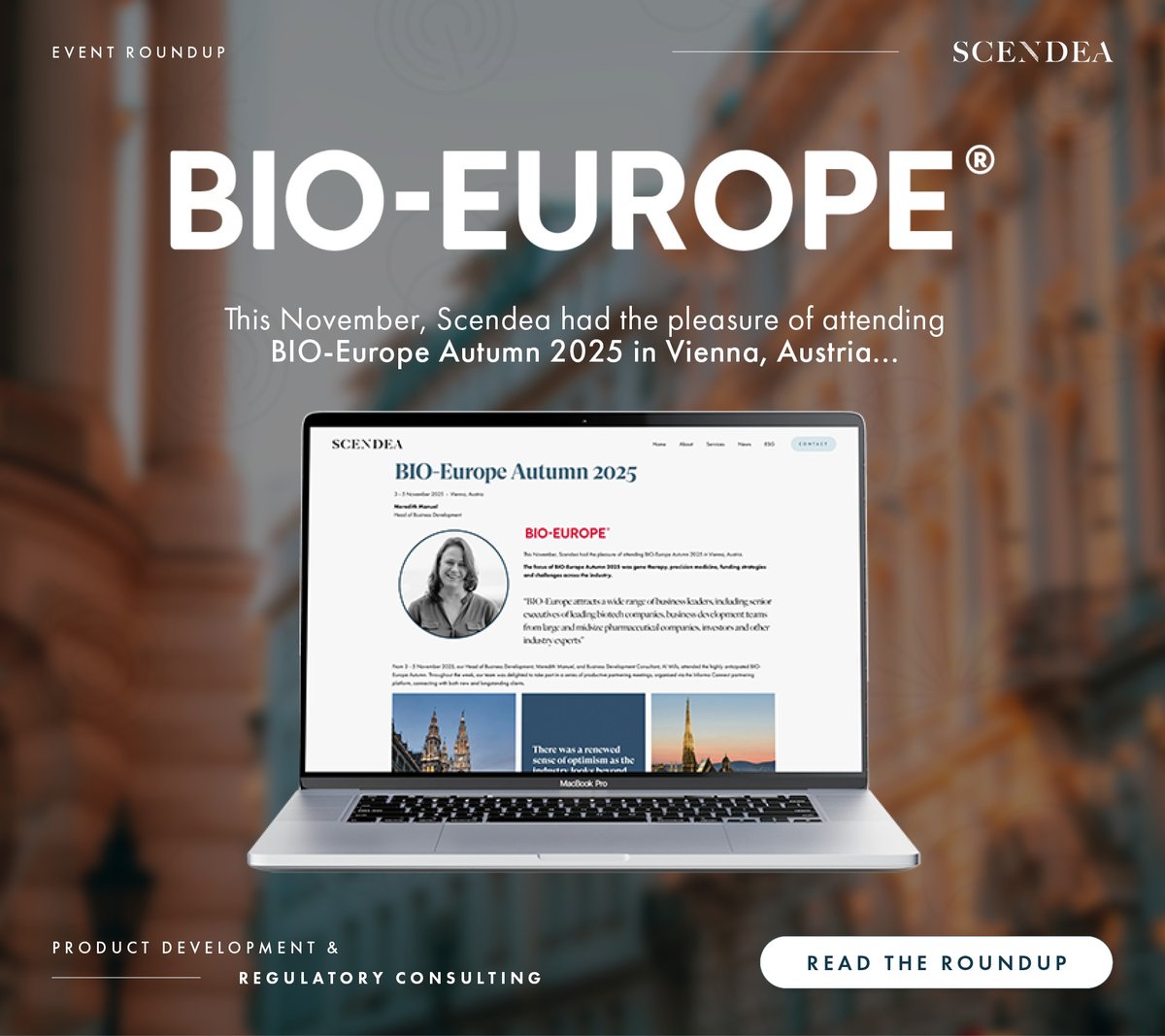
This November, Scendea had the pleasure of attending BIO-Europe Autumn 2025 in Vienna, Austria. Read the full article now, and get in touch to discuss your 2026 product development and regulatory needs with the team: scendea.com/articles/bio-e… - #drugdevelopment #regulatorystrategy
The @EMA_News has updated its Clinical Trials in Human Medicines overview to add a new section on Scientific advice for public health emergencies and threats. - Book a consultation with Scendea’s experts today: scendea.com/contact - #drugdevelopment #regulatoryaffairs




Scendea's Director, Paul Cronin, and Regional BD Manager, Daniel Berg, are travelling across Australia next month, meeting with new and established clients. Explore #Scendea's services and discover how we can support your #medicinal #product development: scendea.com/services

Are you attending the British Neuroscience Association's Festive Symposium? Scendea are delighted to be attending this coming December. Discover how our experts can support your product development journey. Get in touch to book a meeting: scendea.com/contact #FestiveSymposium

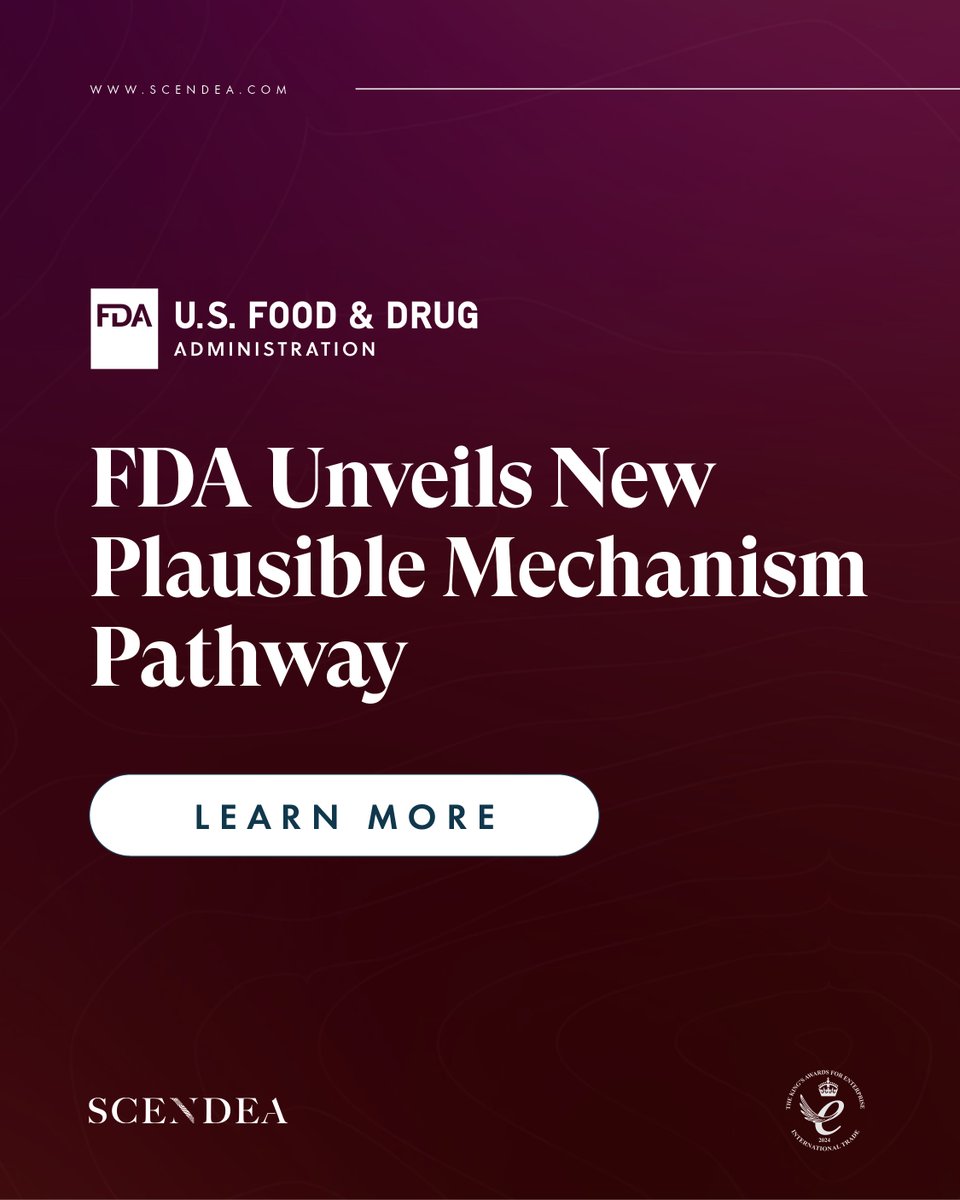
The @US_FDA has published a paper on a plausible mechanism pathway, a #regulatory framework to accelerate approval of bespoke, #patient-specific #therapies when traditional trials are not feasible. - For more information on this guidance, get in touch: scendea.com/contact



Read and download our latest Whitepaper, "Insights on the FDA’s 2024 Guidance: Considerations for the Development of CAR-T Cell Products", authored by Dr Dmitry Zamoryakhin, Scendea's Principal Medical Consultant: scendea.com/articles/consi… - #whitepaper

The @MHRAgovuk has updated its Early Access to Medicines Scheme (EAMS) Overview guidance to revise the “Dates for submission, Day 1 and Day 45” table. - For UK regulatory support, get in touch: scendea.com/contact - #drugdevelopment #earlyaccesstomedicines #MHRA




Yesterday, Scendea had the pleasure of attending the Dutch Life Sciences Conference. Discover how Scendea can support your EU product development and regulatory activities. Get in touch to speak to a member of the team: scendea.com/contact - #EUbiotechevents



Scendea are delighted to be attending Genesis 2025, this December! Secure a meeting with our experts, and discover how Scendea can support your global regulatory and product development strategy: scendea.com/contact - #Genesis #DrugDevelopment #UKbiotechsupport

The @US_FDA has announced the “plausible mechanism” approval pathway for certain personalised rare disease treatments. - Read our new whitepaper to discover how the RDEP could accelerate your path to approval: scendea.com/articles/fdas-… - #whitepaper #drugdevelopment #RDEP




The @US_FDA has released the final guidance on how to prepare a Pre-Request for Designation (Pre-RFD). Get in touch to speak with a member of our team: scendea.com/contact - #preRFD #FDAagencyinteractions




Scendea are delighted to be sponsoring and attending BIO Partnering at JPM Week this coming January! - Schedule a meeting by searching 'Scendea' in the Partnering system or contact us today: scendea.com/contact - #BIOJPM #partnering #regulatoryaffairs #drugdevelopment

The EMA has published a draft guideline on the non-inferiority and equivalence comparisons in clinical trials. For support with your EU regulatory activities, get in touch to speak to Scendea's experts Scendea.com/contact




Scendea's Deputy Chief Medical Officer & Principal Medical Consultant, Dr Maria Beatrice Panico, and Business Development Consultant, Al Mills, are having an excellent time attending LSX Investival Showcase today! - #LSXInvestival #NetworkingEvent #HealthcareInnovation



The @MHRAgovuk has released a policy paper on rare therapies and regulatory considerations. Meet our team of experts and book an introductory call today: scendea.com/contact - #RareDiseaseTherapies #drugdevelopment #UKregulatoryconsiderations




Meet the author from our latest Whitepaper: "Insights on the FDA’s 2024 Guidance: Considerations for the Development of CAR-T Cell Products", Dr Dmitry Zamoryakhin: scendea.com/articles/consi… - #whitepaper #drugdevelopment #CARTtherapies #FDAguidancerecomentations




Are you attending the Dutch Life Sciences Conference? With 1 week to go, now is the perfect opportunity to book a meeting with Scendea and discover how we can support your #drugdevelopment journey! - Get in touch to book a meeting: scendea.com/contact - #DutchLifeScience

United States 趨勢
- 1. Happy Thanksgiving 201K posts
- 2. #StrangerThings5 308K posts
- 3. BYERS 71.9K posts
- 4. Afghan 345K posts
- 5. #DareYouToDeath 168K posts
- 6. DYTD TRAILER 110K posts
- 7. robin 109K posts
- 8. Turkey Day 14.5K posts
- 9. Dustin 63.1K posts
- 10. Vecna 71.5K posts
- 11. Holly 72.6K posts
- 12. Taliban 44.8K posts
- 13. Reed Sheppard 7,300 posts
- 14. noah schnapp 9,685 posts
- 15. Jonathan 77K posts
- 16. Rahmanullah Lakanwal 135K posts
- 17. mike wheeler 11K posts
- 18. Nancy 72.1K posts
- 19. hopper 17.9K posts
- 20. Erica 20.4K posts
你可能會喜歡
-
 King of Prussia
King of Prussia
@ImKingofPrussia -
 BostonTom
BostonTom
@BostonTom6 -
 wmol
wmol
@wmolenzccures -
 Clarivate
Clarivate
@Clarivate -
 D
D
@dwoodru11 -
 cyviwivi
cyviwivi
@cyviwivi -
 Jeff Taitano
Jeff Taitano
@JeffTaitano -
 The Bearded Bigfoot
The Bearded Bigfoot
@klingen_scott -
 matty_xrp
matty_xrp
@matty_otc -
 Salita Singh
Salita Singh
@salita_singh -
 Mr. B
Mr. B
@ConservedSites -
 Casey Talent
Casey Talent
@caseytalent -
 T r i p p y
T r i p p y
@unillusive -
 Mark FTW 🇺🇸
Mark FTW 🇺🇸
@MarkFTW7
Something went wrong.
Something went wrong.