@softwarecpr
@softwarecpr
FDA and ISO/IEC Regulatory Consulting. Onward to higher software quality! Crisis Prevention and Recovery, LLC https://softwarecpr.com
Great internal discussion today on "Architecting for Testability." Many will see that phrase and immediately react with yes! If you know, you know! The best system architects and software architects are considering test interfaces as they design. Have you ever encountered a…
SoftwareCPR is planning a series of training courses in #KualaLumpur, #Malaysia (for Malaysian citizens). Our instructors understand regulatory agency expectations, including US #FDA and EU, and can train your staff accordingly and coach them on how best to articulate and defend…
softwarecpr.com
ISO 13485 and IEC 62304 Training - Kuala Lumpur
SoftwareCPR is planning a series of training courses in Kuala Lumpur, Malaysia (for Malaysian citizens). Our instructors understand regulatory agency
Our ISO 13485 Internal Audit Training Course focuses on both the requirements of ISO 13485:2016 and the audit process and methods(ISO 19011) to ensure the manufacturer or supplier is compliant with the requirements of the standard. Our approach is to educate on the intent and…
softwarecpr.com
Expert Consultation
Crisis Prevention and Recovery LLC does business as SoftwareCPR®. SoftwareCPR® consists of several partners who offer expert consultation in FDA
The international standard, ISO 13485:2016, Medical devices — Quality management systems — Requirements for regulatory purposes, provides the framework of the set of interrelated processes that work to ensure product quality. This international standard is based on a process…
We understand the tension. You get it … we all want to be process focused. Create and maintain a good process, and good outputs will come forth. But you may be the one that faces the auditor or faces the inspector. They ask for evidence that the process was performed. You think,…
@softwarecpr Partner and General Manager, Brian Pate, will be teaching our very popular 3-day #iec62304 #software training course June 24-26, 2025 at one of our clients in #boston. The course is freshly updated with detailed explanation of intent of the standard with richly…
#iec62304 The software system is software safety class A if the software system: - cannot contribute to a hazardous situation; or - can contribute to a hazardous situation which results in acceptable risk after consideration of risk control measures external to that software…
By developing #medicaldevice #software in phases or stages as part of a lifecycle #sdlc, you give yourself the opportunity to “measure.” Why is that important? What do we measure? It is important because it allows you to prove (or argue) that you have accomplished what you…
Refreshing to see such a clearly articulated discussion of #software #design. A good reminder that #softwarequality is "designed-in," not "tested-in." Kudos @flightaware flightaware.engineering/blast-from-the…
Fourth post on software risk analysis. When we consider general #microprocessor failures and concerns, one generally starts with the processor operating core working outward. For example, are we certain that the basic machine instructions actually work? What about the internal…

Are software engineers a commodity that organizations can simply plug-n-play expecting a similar outcome? Unlike many other disciplines, software engineering allows a great deal of freedom within the design space. Two different software engineers can produce significantly…
Third post on software risk analysis. We often get asked about the use of Fault Tree Analysis (#FTA) and Failure Modes and Effects Analysis (#FMEA) methods to aid with analyzing risks associated with #software. Both methods are extremely helpful in the process and I really can't…

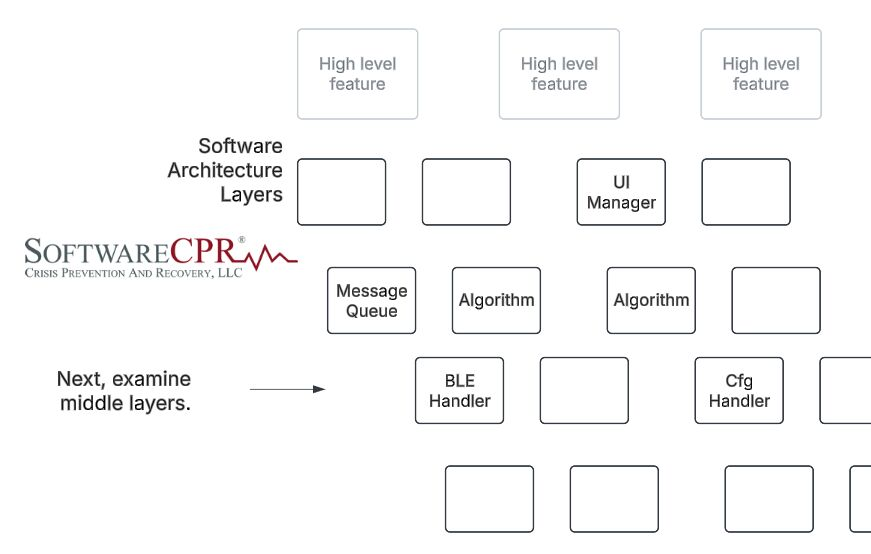
Continuing our thoughts of #software #risk analysis from an #fda and #iec62304 perspective, after starting with high level software functionality related to the intended use, we look next at the middle layers of the software design. Obviously good design practices such as…
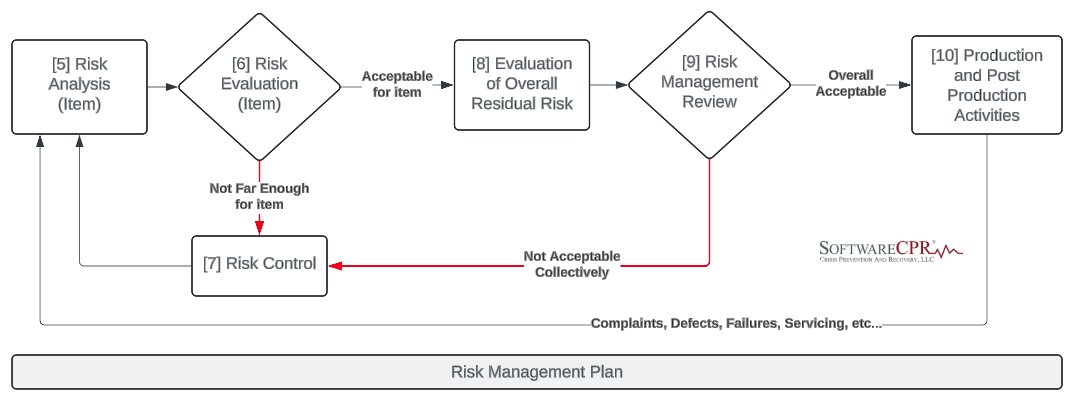
"A risk assessment that includes a risk analysis, risk evaluation, risk control and a benefit-risk analysis (where applicable) should be provided for all device software. For software that is part of a system, a risk assessment should be performed on the system comprising the…

#iso14971 #riskmanagement must start VERY early in the #productrealization process. Even as you discuss and formalize the #intendeduse and claims for the #medicaldevice or #samd, brainstorm the #hazardoussituations associated with the intended use and capture in the preliminary…
One pitfall that we have found over the years among some #medicaldevice companies relates to what they mean by "dry running" test cases and/or "informally" executing #softwareverification tests. The pitfall is that the "testing" that is done is really simplistic and lacks the…
United States 趨勢
- 1. Comey 142K posts
- 2. GeForce Season 2,359 posts
- 3. Everton 90K posts
- 4. Seton Hall 1,429 posts
- 5. Mark Kelly 94.6K posts
- 6. Dorgu 14K posts
- 7. Pickford 3,804 posts
- 8. Opus 4.5 6,400 posts
- 9. Amorim 30.8K posts
- 10. Gueye 22.7K posts
- 11. #MUNEVE 10.9K posts
- 12. Keane 14.9K posts
- 13. Zirkzee 16.9K posts
- 14. Maui 4,291 posts
- 15. Halligan 44.9K posts
- 16. Hegseth 34.9K posts
- 17. UCMJ 14.4K posts
- 18. Pentagon 20K posts
- 19. Creighton 2,280 posts
- 20. Will Wade N/A
Something went wrong.
Something went wrong.