Haystack Project
@HaystackProject
We are committed to the Ultra Rare Disease Community. Bringing together all stakeholders to educate & advocate for reimbursement policies.
Вам может понравиться
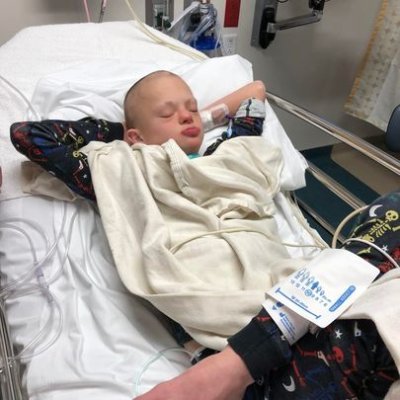
Grateful for the esteemed panelists who made our first Scientific Workshop a success! Your insights on changes to rare disease studies at the FDA were invaluable. Special thanks to Dr. Janet Woodcock for leading the effort! Want to learn more or support Haystack Project? DM us!

CEO of Haystack Project, Kara Berasi, provides public comment at the Advancing Rare Disease Therapies Through an FDA Rare Disease Innovation Hub haystackproject.org/heart-act

Haystack writes CMS regarding what data the agency should consider in negotiating drug prices per the IRAhaystackproject.org/resources-3/20…
This letter to CMS covers the numerous issues with implementing the negotiation provisions in the IRA, especially the impact on rare disease patients of CMS’ (mis)interpretation of “qualified single source drug” (QSSD). haystackproject.org/resources-3/20…
Inside Health Policy highlights Haystack Project’s call for change at recent FDA Listening Session. Conflict of interest rules must accommodate the critical need for rare disease clinician experts on Advisory Committees with appropriate disclosures. haystackproject.org/heart-act
Haystack Project challenges CMS to address low volume/high-cost DRG problem. Read our IPPS comments here. haystackproject.org/resources-3/20…
Haystack Project highlights all the ways Medicare Advantage plans can be improved for rare disease patients haystackproject.org/resources-3/20…
EXTRA! EXTRA! Read All About It; Haystack Project April 2024 Recap static1.squarespace.com/static/5966cc2…
Haystack gives CMS feedback on what our patients think about the MP3’s six model documents. haystackproject.org/resources-3/20…
Great discussion with BMS SVP on Value-Based Arrangements and Rare at Haystack’s Speaker Series… Thank you, Ranjani Durham! haystackproject.org/speaker-series
Kara Berasi, HP’s CEO, will be attending the World Orphan Drug Conference, and participating on a panel on April 25th from 3:55-4:35 PM called “How Policy Moves Forward with Patients at The Center” If you're attending this, please contact Kara to meet up or attend the panel!


Haystack Project submits a comment letter to CMS regarding how to make the new “smoothing” program as patient-friendly for rare disease patients as possible. haystackproject.org/resources-3/20…
Haystack calls out the need to monitor for utilization management, esp. in protected classes, as IRA rolls out. haystackproject.org/resources-3/20…
Excited for Rare Disease Day! The Protect Rare Act (HR 6094) is on the agenda at the Health Subcommittee meeting!! Help us celebrate by asking your Representative to cosponsor the bill here: forms.gle/ax66HPXbpE9Jym…

Energy and Commerce, Health Subcommittee Legislative Hearing Hearing on Rare Disease Bills @RepGuthrie @RepAnnaEshoo @RepLarryBucshon Including our push for Protect Rare HR 6094 Today Thursday, February 29th 10:00am Livestreamed at energycommerce.house.gov/events/health-…
United States Тренды
- 1. Flacco 33.8K posts
- 2. Bengals 50.7K posts
- 3. Bengals 50.7K posts
- 4. Ramsey 11.7K posts
- 5. Tomlin 8,146 posts
- 6. Chase 90K posts
- 7. Max Scherzer 8,143 posts
- 8. #TNFonPrime 3,393 posts
- 9. Ace Frehley 75.1K posts
- 10. Darnell Washington 1,806 posts
- 11. DJ Turner 2,080 posts
- 12. #HereWeGo 8,111 posts
- 13. Cuomo 62K posts
- 14. #PITvsCIN 3,971 posts
- 15. #WhoDey 3,435 posts
- 16. #911onABC 15.3K posts
- 17. Zac Taylor 1,402 posts
- 18. Metcalf 5,476 posts
- 19. Bolton 189K posts
- 20. Sliwa 27.9K posts
Something went wrong.
Something went wrong.