
Intensity Therapeutics
@IntensityInc
Intensity Therapeutics is a clinical stage, biotechnology company whose mission is to create safer and more efficacious cancer therapies.
قد يعجبك
@medpagetoday featured $INTS in its latest oncology roundup, highlighting Phase 1/2 data showing #INT2306achieved 75% disease control & a median OS of 11.9 months in advanced #solidtumors. Proud to see continued recognition of our progress. Read more: bit.ly/4p1XEQi
#IntensityTherapeutics' CEO, Lew Bender, discusses the challenges of current neoadjuvant regimens and how #INT2306 could offer a more targeted, less toxic approach for women with #TNBC. Read the full feature via #DrugDiscovery: bit.ly/4hOkLew $INTS #CancerResearch

Immunohistochemistry (IHC) results following two #INT230-6 doses in the Phase 1/2 study show T-cell infiltration in a sarcoma patient’s tumor compared to the pre-dose biopsy. Seen in 13/14 matched pair biopsies, supporting proof-of-concept for immune activation in tumors. Read…
#IntensityTherapeutics had a great time at the @ctosociety annual meeting with a large number of our Phase 3 investigators who are advancing care for rare and hard-to-treat #sarcomas. $INTS appreciated the chance to engage with other researchers, clinicians, & partners as we…

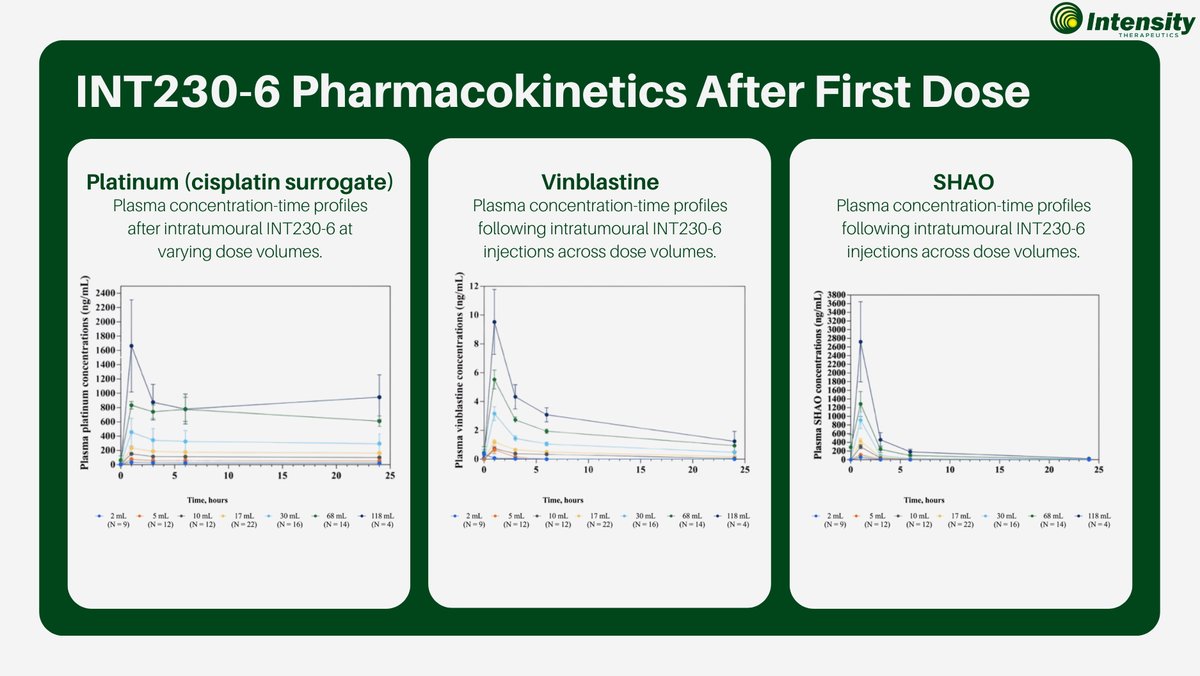
#IntensityTherapeutics’ Phase 1/2 study shows INT230-6 has predictable dose response plasma exposure for cisplatin, vinblastine, and SHAO across dose volumes from 2 to 118 mL, indicating >95% of the active agents remain in the tumor and supporting controlled, consistent delivery…
$INTS Phase 1/2 study shows haematoxylin & eosin staining of a biopsy from a patient with liposarcoma. Darker areas show cancer cells, lighter areas show tumor necrosis. 13 of 14 matched-pair biopsies showed similar patterns. Full @eBioMedicine paper: bit.ly/3LdqWg2
Kaplan-Meier data from #IntensityTherapeutics’ INT230-6 study shows improved survival in advanced #sarcoma. In a heavily pretreated sarcoma patient population using intratumoral INT230-6 alone, the median overall survival (mOS)was 21.3 months. Patients dosed at ≥40% of their…

Check out this interview in @afurtherinquiry on $INTS' intratumoral therapy #INT2306. CEO, Lew Bender, explains how INT230-6 is redefining #cancertreatment, the science behind #DfuseRx, and insights from ongoing #clinicaltrials. Read more: bit.ly/4hFlqim

#IntensityTherapeutics reports Q3 2025 financial results & a corporate update. #INTS plans to file a protocol amendment in the #INVINCIBLE4 study to revise dosing & reinitiate enrollment in Q1 2026. With recent capital raises, $INTS' cash runway now extends through Q1 2027.…

#INTS’ study published in @eBioMedicine shows #INT2306 was well tolerated & demonstrated antitumor activity, immune activation, & abscopal effects. The results support $INTS' approach to treating injected & noninjected tumors. Full paper: bit.ly/3LdqWg2 #eBioMedicine

#IntensityTherapeutics highlights how INT230-6 targets tumors & activates immune cells. Phase 1/2 data in @eBioMedicine show intratumoral dosing triggers T-cell infiltration, abscopal effects, & improved disease control. Read the full manuscript: bit.ly/3LdqWg2 $INTS
$INTS Phase 1/2 of INT230-6 shows safety & efficacy in metastatic/refractory cancers. ≥40% tumor burden dosing: 83% disease control, 18.7-mo median OS & ~20% abscopal effects. @DrElkhoueiry highlights local & systemic potential. @eBioMedicine paper: bit.ly/3LdqWg2

$INTS has entered into a securities purchase agreement with a new long-term fundamental investor for the purchase and sale of 5 million shares of common stock at a purchase price of $0.80 per share for gross proceeds of about $4 million. Read more: bit.ly/433Sr1Q

Tomorrow at 9 AM ET, $INTS will host a live webinar with authors from @uscnorris & CEO @LewBender on the @eBioMedicine study of INT230-6 in advanced solid tumors. Join to hear key insights from the Phase 1/2 trial. Register: bit.ly/3Wu6ObT #eBioMedicine @DrElkhoueiry

In a new Q&A with @ValiantCEO_ , #IntensityTherapeutics' CEO, Lew Bender, shares how $INTS evolved from a basement startup to a late-stage biotech listed on Nasdaq that is advancing its first-in-class #cancer therapies, including INT230-6. Full Q&A: bit.ly/3Jz9ylp #INTS
Over 316,000 new invasive breast cancer cases are expected in the U.S. this year, according to @theNCI. #IntensityTherapeutics is advancing INT230-6 to activate the immune system for hard-to-treat #breastcancers. Learn more: bit.ly/40ipTjJ $INTS #BreastCancerAwareness

$INTS is proud to have our VP of Clinical Operations, Kimberly Guedes, featured as a keynote presenter at the COG Bay Area conference kicking off today. She’ll discuss how stakeholder collaboration accelerates #clinicaltrials, drives innovation, & operational excellence.

#IntensityTherapeutics is excited to attend @avbcancercare's Cancer Research & Innovation Symposium on Oct 21 in NY. CEO, Lew Bender, will join the “Blueprint for Emerging Start-ups” panel at 9:30am ET to share insights on funding, pitching, & early-stage growth. #GCRIS2025

#INTS is honored to be shortlisted for @LifeSciEvents' #FierceInnovationAwards: Life Sciences Edition 2025 in Drug Delivery! This recognizes $INTS's innovation in cancer research & lead candidate INT230-6. All finalists: bit.ly/4qdpFFO #FierceLifeSciencesAwards

United States الاتجاهات
- 1. Cheney 43.5K posts
- 2. Nano Banana Pro 15.3K posts
- 3. First Take 43.8K posts
- 4. #AcousticPianoSnowGlobe 1,990 posts
- 5. Cam Newton 2,328 posts
- 6. Stephen A 37.2K posts
- 7. SEDITIOUS BEHAVIOR 13.1K posts
- 8. FINAL DRAFT FINAL LOVE 371K posts
- 9. Sedition 88.6K posts
- 10. Trump and Vance 29.5K posts
- 11. #XboxPartnerPreview 1,661 posts
- 12. #LoveDesignFinalEP 339K posts
- 13. Bush 52.7K posts
- 14. Treason 57.8K posts
- 15. #TSTheErasTour 1,619 posts
- 16. Eddie Hennessy N/A
- 17. Husqvarna 1,106 posts
- 18. Tides of Annihilation N/A
- 19. Godzilla 22.1K posts
- 20. #WeekndTourLeaks 1,121 posts
قد يعجبك
-
 jive_turkey
jive_turkey
@jivetur88775834 -
 Jacob Plieth
Jacob Plieth
@JacobPlieth -
 Flagship Pioneering
Flagship Pioneering
@FlagshipPioneer -
 SEED Innovations Limited
SEED Innovations Limited
@SEEDInnov -
 Ed McDermott
Ed McDermott
@edmcdermott12 -
 Ant_Man 🗽
Ant_Man 🗽
@online_antz -
 Jam4U
Jam4U
@Jam4u_75 -
 1971bod
1971bod
@1971bod -
 RᴇᴍᴇᴍʙᴇʀɪɴɢTʜᴇFᴜᴛᴜʀᴇ™
RᴇᴍᴇᴍʙᴇʀɪɴɢTʜᴇFᴜᴛᴜʀᴇ™
@RTF83 -
 Mathew Barker
Mathew Barker
@MathewBarker11 -
 📈 🐝 Small Company Champion 🏴🇬🇧
📈 🐝 Small Company Champion 🏴🇬🇧
@LEMMINGINVESTOR -
 Allan Shaw
Allan Shaw
@Allan_L_Shaw -
 superyachter
superyachter
@Superyotter
Something went wrong.
Something went wrong.















































































































