#medicaldevicesoftware نتائج البحث
Certified according to DIN EN ISO13485: Development and sales for #medicalsoftwaresolutions & #medicaldevicesoftware

#medicaldevicesoftware #MedTech #MedicalDevices #healthcare #BigPharma #technology #MedicalDevice #biotechnology #medtechcourses #medtwitter #publicationwriting #CERwriting #medicalwriting #medicalwritingcourse #freelancemedicalwriting #workshops #webinar #medcomms #healthtech

"Bond is at the heart❤️of the lateral flow industry!" - Phil Groom, Commercial Director. We're elevating diagnostic tests to the next level via custom App development and Cloud connectivity! #LateralFlowWorkshop2019 #lfw2019 #medicaldevicesoftware #healthtech #biotech

Join us later today in a #webinar in #MedicalDeviceSoftware Development. Don't miss it! ow.ly/YRqp3014K3u

Meet us at the Medical Device Software Development Summit Europe 2023. #medicaldevicesoftware #embeddedsoftware

In this article, our QA Department Manager Kristina Sivchenko explores the specifics of #medicaldevice #softwaretesting and delves into the details of the required testing protocols and the impact they have on the development of #medicaldevicesoftware. hubs.la/Q02h1rkR0
How can your embedded team dramatically reduce the risk of #medicaldevicesoftware development? Find out in this guide! Learn more >> prsft.co/3wMqGdG

MPD Training Course: Medical Device Software, 7th Feb @MedilinkEM: This one-day course will cover #Regulatory insights into #MedicalDeviceSoftware. Find out what you need to do to comply with the Regulation. EARLY BIRD DISCOUNT (Book before 18 January): bit.ly/2Cm3c2g

Increase Efficiency and Innovation in #MedicalDeviceDevelopment! Whether you need the full-cycle development of #medicaldevicesoftware or only medical device software consulting, our experienced team of business analysts, developers, Let’s talk: bit.ly/3rHAdjI

CorTechs Labs - Brain Imaging Analysis Read more: bit.ly/2QRE9gu #BrainImagingAnalysis #CorTechsLabs #MedicalDeviceSoftware

Our FDA and regulated environment experience helped us improve the safety of our client’s ambulatory infusion pump: sep.fyi/2ts7Fje #embeddeddevelopment #medicaldevicesoftware
Developing Compliant, Innovative, & Secure #MedicalDeviceSoftware! ISO 9001 certification and 11+ years of #healthcare IT experience back up Dash Technologies’ skills in creating secure #software for #medicaldevices and SaMD. To learn more 👉 bit.ly/2NKtapW #OpenEMR

MPD Training Course: Medical Device Software, 4th June @MedilinkEM: This one-day course will cover #Regulatory insights into #MedicalDeviceSoftware. Find out what you need to do to comply with the Regulation. EARLY BIRD DISCOUNT (book before 14th May): bit.ly/2Cm3c2g

Learn about the #IEC62304 safety standard for developing #medicaldevicesoftware and how to achieve #compliance. parasoft.com/blog/what-is-i… #software #softwaredevelopment #softwaretesting #medicaldevice

Our extensive embedded and FDA device development experience to the table as we improved the safety of our client’s ambulatory infusion pump. Check out the story here: sep.fyi/2SL6Lc4 #embeddeddevelopment #medicaldevicesoftware
The #MDR's entry into force was postponed by a year, so now the medical device manufacturers have time to prepare for the change even better. How MDR affects #MedicalDeviceSoftware processes? Read more from Juha's blog! hubs.ly/H0q8NbM0 #medicaldevice #softwaredevelopment

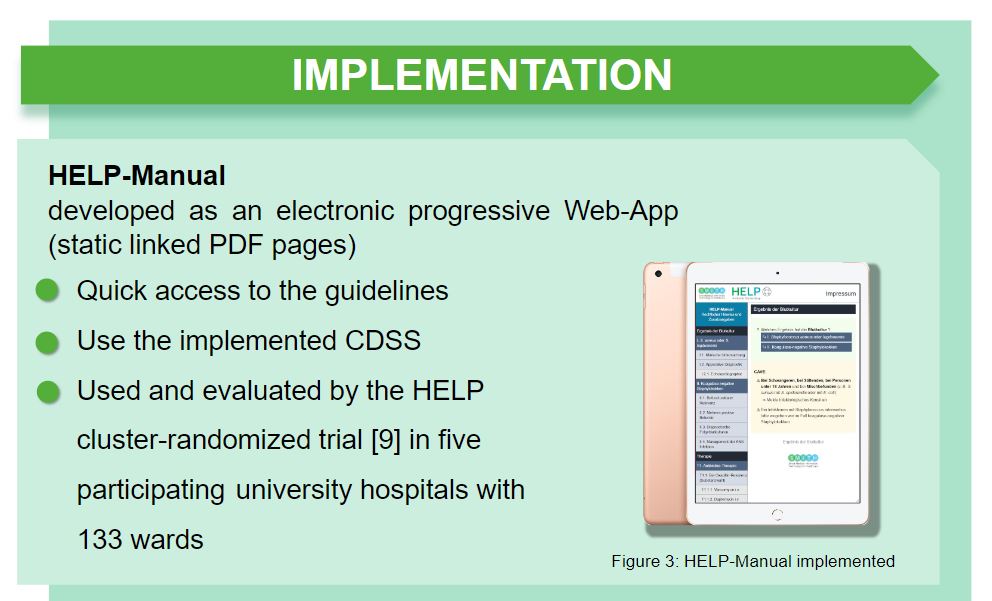
Muss es immer #MedicalDeviceSoftware sein? Unsere Lessons learned aus @SMITHKonsortium-HELP gibt es als #ePoster auf der #gmdsTMF2022 Diskussion: 23.08. 17:30h Networking Area doi.org/10.3205/22gmds… @RWTH @UniklinikAachen @MII_Germany @BMBF_Bund @MyriamLipprandt @RainerRohrig

IEC 62304 compliance ensures the safe development of #medicaldevicesoftware. What do you think is the best method and first line of defense to fix bugs or issues? parasoft.com/blog/what-is-i… #software #softwaredevelopment #softwaretesting #medicaldevice #iec62304

Schon gewusst? CONZE entwickelt u.a. Software zur dentalen Rekonstruktion. Daher feiern wir unsere Kunden am morgigen „Tag des Zahnarztes“ und danken für ihren unermüdlichen Einsatz! #Medizintechnik #MedicalDeviceSoftware #Medizinproduktesoftware #DentalSoftware

Medical device developers need to ensure that the software meets the requirements of new legislation focused on cybersecurity. Here are some best practices that can help. ter.li/g06jm6 #MedicalDeviceSoftware #Cybersecurity

'EU MDR puts software safety first' Register now for EU Regulation and Quality Management of Medical Device Software MasterClass by Richard Tully & Heidi Naderi: bit.ly/3Ss8uR4 & bit.ly/3GFJinx #glceurope #masterclass #medicaldevicesoftware #qualitymanagement



'Compliance drives safe innovation' Register now for EU Regulation and Quality Management of Medical Device Software MasterClass by Richard Tully & Heidi Naderi: bit.ly/3DToE2f & bit.ly/43rBhfp #glceurope #masterclass #medicaldevicesoftware #qualitymanagement

The overarching goal is to enhance transparency and consistency, supporting comprehensive descriptions, thorough risk assessments, and interpretations of classification approaches across global regulatory jurisdictions. 2/4 #SAMD #MedicalDeviceSoftware #Healthtech
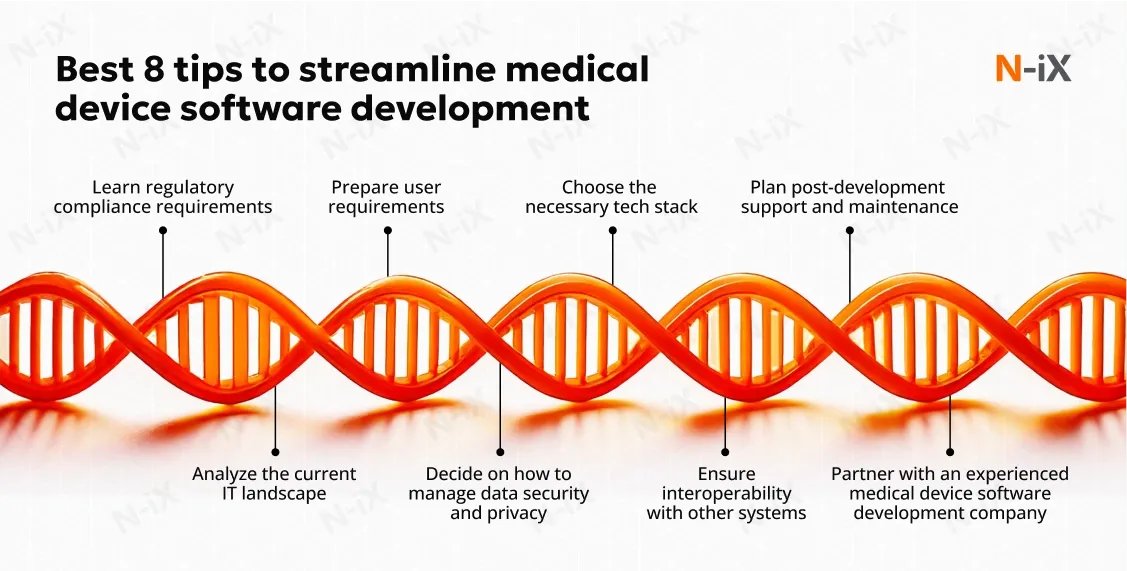
Developing #medicaldevicesoftware can be a maze. Our 8 expert tips will guide you through the complexities. 🧭 Learn how to navigate regulations, integrations, and more to achieve optimal outcomes for your projects. ➡️ n-ix.com/medical-device…
Is your software ready for the moment? In the fast-paced MedTech industry, flawless performance under real-world conditions is vital. Read more here: (bluegoatcyber.com/blog/medical-d…) #MedicalDeviceCompanies #HealthcareInnovation #MedicalDeviceSoftware #BlueGoatCyber #MedTech

Ensure Compliance with IEC 62304 – Medical Device Software Training Developing Medical Device Software According to IEC 62304 Date: Oct 03-04, 2024 Enroll Now: hubs.li/Q02QKCkf0 #medicaldevicesoftware #IEC62304 #softwaretraining #medicaltechnology #medicaldeviceindustry

SaMD – USA and EU Approach Differences Explore the key differences between US FDA and EU MDR regulations for Software as a Medical Device (SaMD) Read more: ddismart.com/blog/samd-usa-… #EUMDR #MedicalDeviceSoftware #MedicalRegulations #MedicalSoftware

Revolutionize healthcare with cutting-edge medical device software! Dive into the complete process of developing medical device software, from ideation to launch.Check out the blog now👇 whitelotuscorporation.com/a-complete-gui… #medicaldevicesoftware #SoftwareDevelopment #whitelotuscorporation
What's next in #medicaldevicesoftware? Our guide explores the latest technological advancements, highlighting innovative trends shaping the future. Stay ahead of the curve with insights into best practices and overcoming common challenges. cabotsolutions.com/a-guide-to-med…
cabotsolutions.com
A Guide to Medical Device Software Development
Whether you're a seasoned developer, a healthcare professional, or simply curious about the future of medicine, this guide is your roadmap to understanding the fascinating world of medical device...
Wanna tackle the tricky parts of medical device regulations? Read the second part of our RegProof™ blog series: Laying the foundation for calm compliance 👉 brnw.ch/21wKbST #regulations #medicaldevicesoftware #impactthatlasts
Exciting news for @QIAGENBiox! The clinical decision support platform QIAGEN Clinical Insight Interpret is now IVDR certified in Europe. Learn more 👉 buff.ly/3V5iyBW #NGS #IVDR #MedicalDeviceSoftware

Exciting news for @QIAGEN! The clinical decision support platform QIAGEN Clinical Insight Interpret is now IVDR-certified in Europe. Learn more ⬇️ #NGS #IVDR #MedicalDeviceSoftware
Do you need support securing compliance and the best #regulatory strategy for your #medicaldevices? Or do you need support characterizing your #medicaldevicesoftware and defining which regulatory requirements to fulfil? Reach out to us ☞ [email protected] 6/6
For manufacturers and developers of #medicaldevicesoftware #MDSW in #Switzerland. 🇨🇭 @Swissmedic_ published an updated version of the information MDSW including revisions relating to regulatory requirements and obligations of economic operators. 1/6 swissmedic.ch/dam/swissmedic…

Developing Medical Device Software According to IEC 62304 Date: Apr 29-30, 2024 Enroll Now: hubs.li/Q02v1dnX0 #MedicalDeviceSoftware #IEC62304 #SoftwareDevelopment #MedicalTechnology #ComplianceTraining #HealthcareTech #RegulatoryCompliance #QualityAssurance #Software #IEC

#medicaldevicesoftware #MedTech #MedicalDevices #healthcare #BigPharma #technology #MedicalDevice #biotechnology #medtechcourses #medtwitter #publicationwriting #CERwriting #medicalwriting #medicalwritingcourse #freelancemedicalwriting #workshops #webinar #medcomms #healthtech

Certified according to DIN EN ISO13485: Development and sales for #medicalsoftwaresolutions & #medicaldevicesoftware

MPD Training Course: Medical Device Software, 7th Feb @MedilinkEM: This one-day course will cover #Regulatory insights into #MedicalDeviceSoftware. Find out what you need to do to comply with the Regulation. EARLY BIRD DISCOUNT (Book before 18 January): bit.ly/2Cm3c2g

MPD Training Course: Medical Device Software, 4th June @MedilinkEM: This one-day course will cover #Regulatory insights into #MedicalDeviceSoftware. Find out what you need to do to comply with the Regulation. EARLY BIRD DISCOUNT (book before 14th May): bit.ly/2Cm3c2g

"Bond is at the heart❤️of the lateral flow industry!" - Phil Groom, Commercial Director. We're elevating diagnostic tests to the next level via custom App development and Cloud connectivity! #LateralFlowWorkshop2019 #lfw2019 #medicaldevicesoftware #healthtech #biotech

Muss es immer #MedicalDeviceSoftware sein? Unsere Lessons learned aus @SMITHKonsortium-HELP gibt es als #ePoster auf der #gmdsTMF2022 Diskussion: 23.08. 17:30h Networking Area doi.org/10.3205/22gmds… @RWTH @UniklinikAachen @MII_Germany @BMBF_Bund @MyriamLipprandt @RainerRohrig


Join us later today in a #webinar in #MedicalDeviceSoftware Development. Don't miss it! ow.ly/YRqp3014K3u

"Plataformas de participación del paciente en la monitorización del paciente crónico" ▶️ bit.ly/3Mh6LM9 📝 Por @NataliadelaFig1, cofundadora de @Genesis_Biomed El artículo incluye un árbol de decisiones que revela cuando se deberán certificar como #MedicalDeviceSoftware

CorTechs Labs - Brain Imaging Analysis Read more: bit.ly/2QRE9gu #BrainImagingAnalysis #CorTechsLabs #MedicalDeviceSoftware

Exciting news for @QIAGENBiox! The clinical decision support platform QIAGEN Clinical Insight Interpret is now IVDR certified in Europe. Learn more 👉 buff.ly/3V5iyBW #NGS #IVDR #MedicalDeviceSoftware

In this article, our QA Department Manager Kristina Sivchenko explores the specifics of #medicaldevice #softwaretesting and delves into the details of the required testing protocols and the impact they have on the development of #medicaldevicesoftware. hubs.la/Q02h1rkR0

Is your software ready for the moment? In the fast-paced MedTech industry, flawless performance under real-world conditions is vital. Read more here: (bluegoatcyber.com/blog/medical-d…) #MedicalDeviceCompanies #HealthcareInnovation #MedicalDeviceSoftware #BlueGoatCyber #MedTech

Need to know how to meet the challenges of achieving cost-effective certification in #MedicalDevice software? Then read our white paper: ldra.com/documentviewer… #MedicalDeviceSoftware #MedicalTechnology #LDRA

French regulator for medicines and health products #ANSM sets up committee to combat cyber-attacks on #medicaldevicesoftware #jetmachine #cyberattack ow.ly/N8fR30gKYDV

Learn about the #IEC62304 safety standard for developing #medicaldevicesoftware and how to achieve #compliance. parasoft.com/blog/what-is-i… #software #softwaredevelopment #softwaretesting #medicaldevice

Medical device developers need to ensure that the software meets the requirements of new legislation focused on cybersecurity. Here are some best practices that can help. ter.li/g06jm6 #MedicalDeviceSoftware #Cybersecurity

Developing Compliant, Innovative, & Secure #MedicalDeviceSoftware! ISO 9001 certification and 11+ years of #healthcare IT experience back up Dash Technologies’ skills in creating secure #software for #medicaldevices and SaMD. To learn more 👉 bit.ly/2NKtapW #OpenEMR

Meet us at the Medical Device Software Development Summit Europe 2023. #medicaldevicesoftware #embeddedsoftware

How can your embedded team dramatically reduce the risk of #medicaldevicesoftware development? Find out in this guide! Learn more >> prsft.co/3wMqGdG

Developing Medical Device Software According to IEC 62304 Date: Apr 29-30, 2024 Enroll Now: hubs.li/Q02v1dnX0 #MedicalDeviceSoftware #IEC62304 #SoftwareDevelopment #MedicalTechnology #ComplianceTraining #HealthcareTech #RegulatoryCompliance #QualityAssurance #Software #IEC

Something went wrong.
Something went wrong.
United States Trends
- 1. #AEWDynamite 10.2K posts
- 2. Donovan Mitchell 3,137 posts
- 3. #Survivor49 1,842 posts
- 4. UConn 5,352 posts
- 5. #CMAawards 2,188 posts
- 6. Arizona 29.8K posts
- 7. #SistasOnBET N/A
- 8. Jaden Bradley N/A
- 9. #cma2025 N/A
- 10. Shelton 2,289 posts
- 11. Jarrett Allen 1,331 posts
- 12. Savannah 4,204 posts
- 13. Nick Allen N/A
- 14. FEMA 34.1K posts
- 15. Koa Peat N/A
- 16. Sheila Cherfilus-McCormick 27.5K posts
- 17. Sengun 3,635 posts
- 18. Zach Top N/A
- 19. Jay Huff N/A
- 20. Ricochet 1,979 posts