OrgChemFront
@OrgChemFront
Organic Chemistry Frontiers is an international high impact journal for cutting-edge researches from all disciplines of organic chemistry | 2023 IF - 4.6
قد يعجبك
👉Check out recent #research: Synergistic catalytic allenylic alkylation for #stereodivergent construction of allenes bearing 1,3-axial and central chirality by Xiaohua Liu's team. 🔗doi.org/10.1039/D5QO00…

🔓#OpenAccess #metalcatalyst Read 1,2 Wagner–Meerwein shift in aza-Nazarov cyclization: Bi(iii)-catalyzed substrate-dependent divergent synthesis of highly substituted pyrroles and indenes by Rishikesh Narayan's team. 🔗doi.org/10.1039/D5QO00…

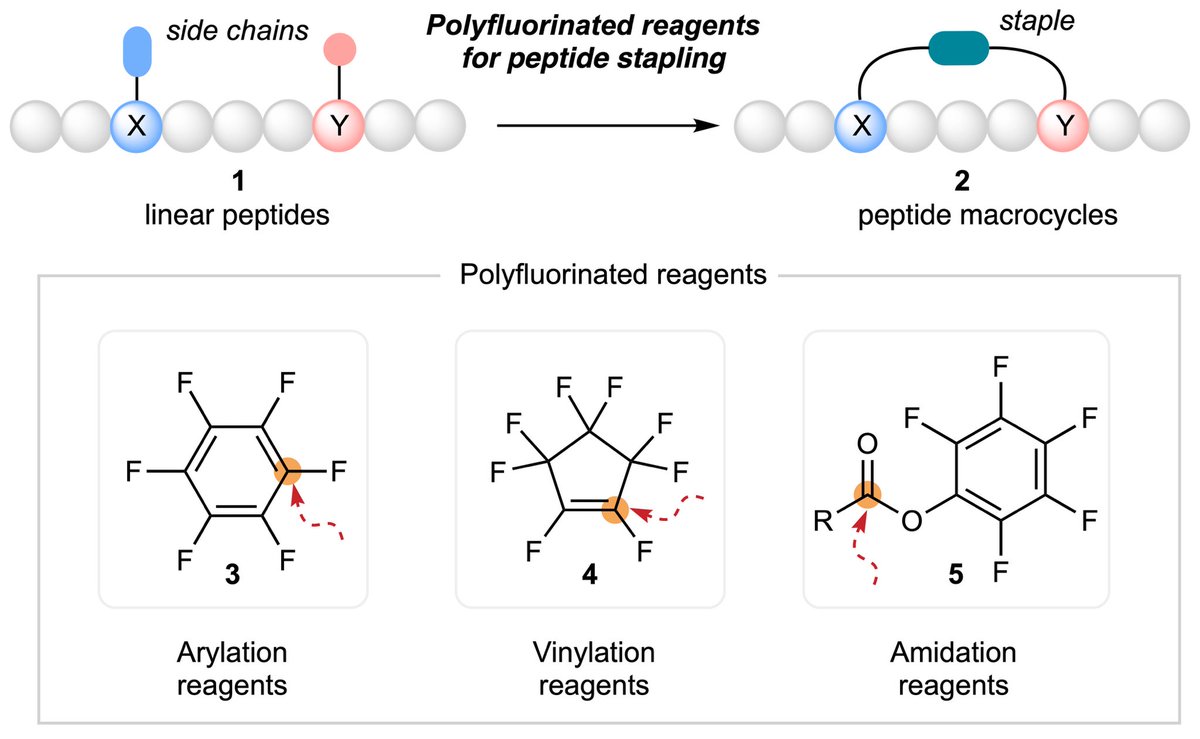
👉Check out recent #review: Polyfluorinated reagents for peptide stapling, which focused on three major classes of polyfluorinated reagents: #rylation, #vinylation, and #amidation reagents. 🔗doi.org/10.1039/D5QO00…

🔓#OpenAccess #HAT #SET Check out #research: Beyond HAT: harnessing TBADT for photocatalyzed Giese-type C(sp³)–C(sp³) bond formation through reductive decarboxylation by Maurizio Fagnoni et al. @unipv 🔗doi.org/10.1039/D5QO00…

🔥Recent #research by EiC Shengming Ma's team: Copper-catalyzed aerobic oxidation of aldehydes to carboxylic acids, in which an aerobic #oxidation of #aldehydes to #carboxylic acids using O₂ or air as the oxidant has been developed. 🔗doi.org/10.1039/D5QO00…

👉Check out recent #research Conformational preference of N-difluoromethylated amides: contributions of hydrogen-bonding, #steric, and #stereoelectronic effects by Ryu Yamasaki et al. @spu_kouhou 🔗doi.org/10.1039/D5QO00…

👉Read the #review regarding the #oxetane core #tolerance towards the reaction conditions of the typical toolbox: Oxetane as a part of modern medicinal chemistry toolbox: the advanced synthesis of 3,3-disubstituted building blocks 🔗doi.org/10.1039/D5QO00…

👉Read recent #research meta-Selective thiofluoroalkylation of substituted pyridines via Zincke imines table by @willcdhartley, Omar Boutureira et al. @universitatURV 🔗doi.org/10.1039/D5QO00…

Back cover of Issue 11 showcases research from Bappaditya Gole 𝘦𝘵 𝘢𝘭. "Unveiling stereoselective ladders viaphoto-oligomerization of a diazaanthracene macrocycle" #Free_to_read 🔗 doi.org/10.1039/D5QO00…

Inside cover of Issue 11 presents "Palladium-catalyzed chemoselective decarboxylative coupling of alkynyl carboxylic acids with halogenated aryl triflates" by Chau Ming So 𝘦𝘵 𝘢𝘭. #Free_to_read 🔗 doi.org/10.1039/D5QO00…

🔔Issue 11 is available! Please read the cover article: "Supramolecular catalysis in the dearomative Michael addition involving nitro-group-activated benzofurans" by Anna Skrzyńska, Łukasz Albrecht 𝘦𝘵 𝘢𝘭. #Free_to_read 🔗doi.org/10.1039/D5QO00…

👉Recent #research clarifies the synergistic roles of Ni and Al: Mechanistic insights into Ni–Al co-catalyzed alkyne carbophosphination enabled by C–P bond activation by Congcong Huang, Yishi Lia and Juan Li. @jnu1906 🔗doi.org/10.1039/D5QO00…

🔥A #hot #review article by @huangx513's team: Recent advances in repurposing natural enzymes for new-to-nature asymmetric #photobiotransformations, which is also included in the Emerging Investigator Series #EMI of OCF. 🔗doi.org/10.1039/D5QO00…

Check out our latest review discussing how to make peptide macrocycles using fluorinated reagents. @OrgChemFront pubs.rsc.org/en/content/art…
👉Read #review article Advancements and perspectives toward radical Truce–Smiles-type rearrangement by Yuxi Wang, Chao Shu et al. 🔗doi.org/10.1039/D5QO00…

🔥Read #hot #review article regarding: Visible light-mediated #halofunctionalization of alkenes for the synthesis of vicinally functionalized organohalides. By Kanta Pajujantaro, Ying Chena and Gong-Qing Liu. #NTU 🔗doi.org/10.1039/D5QO00…

🔥Recent #hot article: Enantioselective catalytic Urech hydantoin synthesis by Yu-Ping He's team, in which an example of asymmetric catalytic UHS is disclosed. Check it out if you are interested! 🔗doi.org/10.1039/D5QO00…

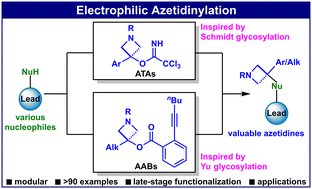
Excited to share our latest work published in @OrgChemFront 🥳🥳🥳. We report two new reagents—ATAs and AABs—inspired by the milestone Schmidt and Yu glycosylation strategies, enabling the direct azetidinylation of diverse nucleophiles at any stage. pubs.rsc.org/en/content/art…
United States الاتجاهات
- 1. Klay 19.1K posts
- 2. #AEWFullGear 68.9K posts
- 3. Lando 95.6K posts
- 4. McLaren 39.4K posts
- 5. #LasVegasGP 180K posts
- 6. LAFC 14.9K posts
- 7. Samoa Joe 4,560 posts
- 8. gambino 2,046 posts
- 9. Hangman 9,586 posts
- 10. Swerve 6,259 posts
- 11. Ja Morant 8,314 posts
- 12. #Toonami 2,738 posts
- 13. Bryson Barnes N/A
- 14. #byucpl N/A
- 15. Max Verstappen 50.2K posts
- 16. Utah 23.9K posts
- 17. Benavidez 15.6K posts
- 18. Mark Briscoe 4,337 posts
- 19. Fresno State N/A
- 20. Kimi 37.1K posts
قد يعجبك
-
 Besset Tatiana
Besset Tatiana
@BessetTatiana -
 Organic Syntheses
Organic Syntheses
@OrgSynth -
 KoenigChemistry
KoenigChemistry
@ChemistryKoenig -
 Martin Group
Martin Group
@MartinLab_ICIQ -
 Noel Research Group
Noel Research Group
@NoelGroupUvA -
 Rovis Group
Rovis Group
@RovisGroup -
 Waser Group
Waser Group
@LcsoLab -
 AggarwalLab
AggarwalLab
@AggarwalLab -
 Morandi Lab
Morandi Lab
@morandilab -
 Silvi Research Group
Silvi Research Group
@SilviResearch -
 Morrill Group
Morrill Group
@MorrillGroup -
 Krische Lab
Krische Lab
@KrischeLab -
 Lutz Ackermann
Lutz Ackermann
@aztul -
 Dixon Group
Dixon Group
@dixon_group -
 Gouverneur Group
Gouverneur Group
@GouverneurGroup
Something went wrong.
Something went wrong.