#fdaapproval نتائج البحث
🚨 FDA approves Zepzelca (lurbinectedin) in combination with Tecentriq or Tecentriq Hybreza for maintenance treatment of extensive-stage SCLC after first-line therapy clinicaloncology.com/a/fzIBAA/t #SCLC #LungCancer #FDAApproval

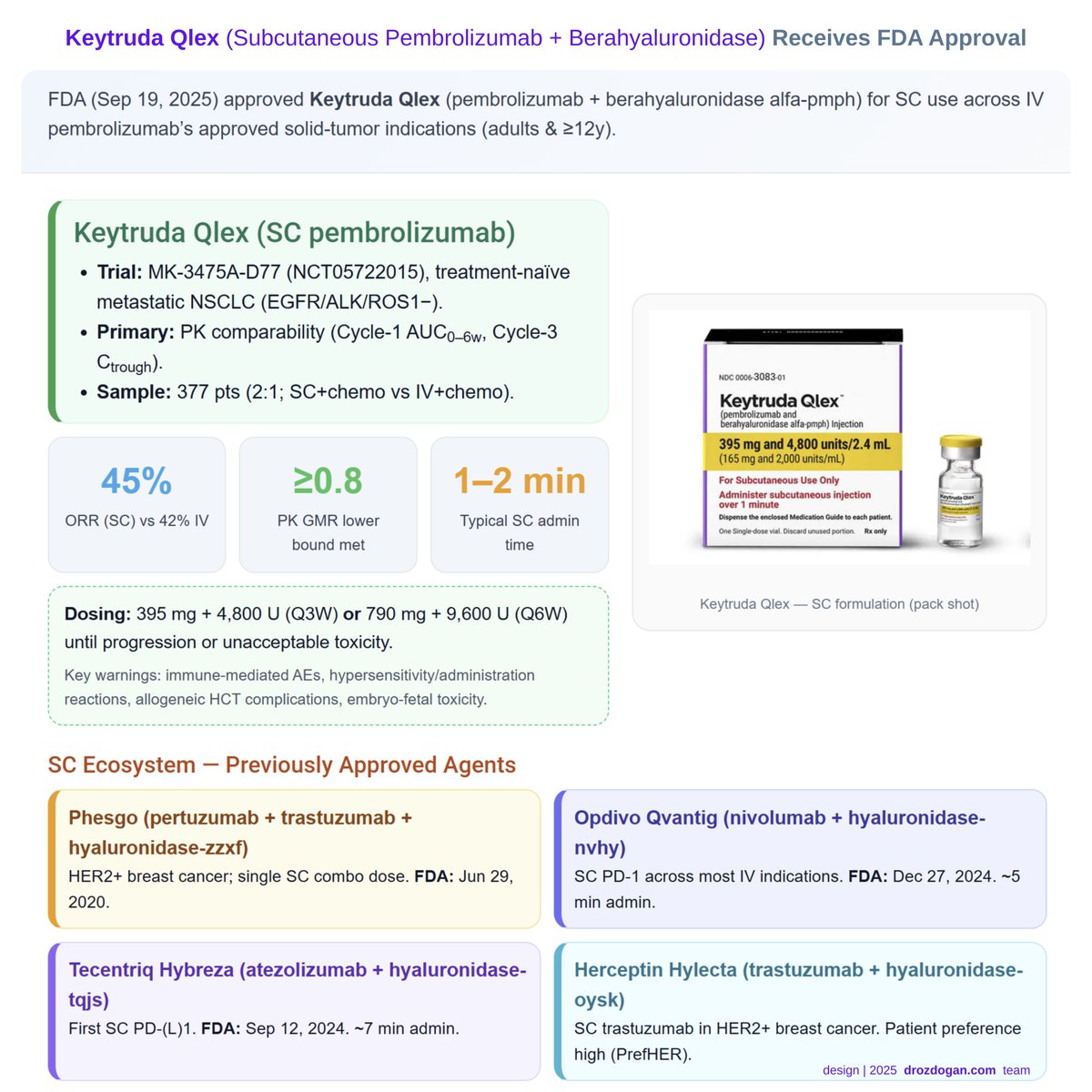
🚨 #FDA approves subcutaneous pembrolizumab (Keytruda Qlex). PK comparability met; ORR 45% SC vs 42% IV in MK-3475A-D77 trial ~1–2 min admin—logistics win, efficacy preserved. #FDAApproval #Oncology #Immunotherapy #CancerResearch #NSCLC #Keytruda #Subcutaneous
The @US_FDA has approved cemiplimab-rwlc for the adjuvant treatment of adults with cutaneous squamous cell carcinoma #skincancer #FDAApproval based on findings from the C-POST study - Median DFS not reached vs 49.4 months w/ placebo (HR = 0.32; P < .0001) ascopost.com/news/october-2…

On August 8, 2025, the FDA grants accelerated approval to zongertinib for non-squamous NSCLC with HER2 TKD activating mutations. Learn more about Beamion LUNG-1 (NCT04886804) 👉 clin.larvol.com/trial-detail/N… #LARVOL #FDAApproval #CancerResearch #Oncology #ClinicalTrials #CancerData…

🌟🇺🇸 #FDAApproval Alert🚨 📅 Aug 6, 2025 ➡️ The FDA has granted accelerated approval to dordaviprone (Modeyso) for H3 K27M-mutant diffuse midline glioma in pts ≥1 y/o with progression after prior therapy. This is the first systemic therapy approved for this indication. 🔬 🧪…

FDA approves Modeyso (dordaviprone), the first therapy for recurrent H3 K27M-mutant diffuse midline glioma. Learn how this oral small molecule could impact patients with this aggressive brain cancer at buff.ly/1XZWQwH by @soumya2390. #FDAApproval #Modeyso #Dordaviprone…
📢Darolutamide FDA-approved for de novo mCSPC Prostate Cancer on 6/3/25! ✅10-yr timeline: 2015 (Docetaxel HR 0.73) → 2025 (Darolutamide OS HR 0.81). 📉rPFS HR 0.54. Trial populations matter! 📰In-Depth Article: oncologytube.com/darolutamide-f… #mCSPC #FDAApproval #OncoTwitter…
The CGuard® Prime Carotid Stent System is now FDA approved! Hear from Marvin Slosman, CEO of InspireMD, as he shares a special announcement on this major milestone—the next generation of carotid artery stenting has officially arrived in the U.S. #FDAApproval #CGuardPrime
The FDA's approval of a low-cost generic abortion pill is a game-changer. What does this mean for healthcare access? #HealthcareReform #FDAApproval #morals? #podcast #news #PodMatch #videopodcast Justadudepodcast.com apple.co/3DqfDh9 spoti.fi/3DFZaVZ…
Lab-grown salmon got the FDA greenlight. 🐟 Rejuran didn’t—yet. Derived from salmon DNA, Rejuran could be the next big thing in skin repair. Food tech may be paving the way for aesthetic biotech. 🎥 youtu.be/xvs2pkirVp8 #Rejuran #FDAApproval #SalmonDNA #SkinRepair…
The FDA has approved a clinical study of an implantable device designed to stabilise blood pressure in people with spinal cord injury. 🔗 Full story: buff.ly/yWVWP5Z #SpinalInjury #NeuroTech #FDAApproval #ARCIM #SCIResearch

The FDA have approved the subcutaneous administration of the powerhouse drug Keytruda/pembrolizumab, which should improve treatment options for patients with solid cancers. #FDAApproval #ClinicalResearch #Cancer #Immunotherapy medpagetoday.com/hematologyonco…
The @US_FDA has approved lurbinectedin + atezolizumab OR atezolizumab + hyaluronidase-tqjs as maintenance for adults with ES-SCLC without progression on first-line atezolizumab ± hyaluronidase-tqjs, carboplatin, etoposide #FDAApproval #SCLC ascopost.com/news/october-2…

The @US_FDA approved estrogen receptor antagonist imlunestrant for pts with ER+/HER2– ESR1-mutant advanced/metastatic breast cancer with disease progression after ≥1 endocrine therapy ➡️Companion diagnostic also approved ascopost.com/news/september… #BCSM #BreastCancer #FDAApproval

From new @US_FDA approvals, to promising clinical trial results, explore @TargetedOnc's weekly roundup of the latest breakthroughs in oncology: bit.ly/42XCyK4 #ClinicalTrials #CancerResearch #FDAApproval #Oncology
A big step forward for #HeartFailure care! 💧💉 The new FDA-approved subcutaneous diuretic offers patients a simpler, less invasive option to manage edema potentially reducing hospital visits and improving quality of life. 👏 #CardioTwitter #FDAApproval
The @US_FDA approved selumetinib granules and capsules for pediatric pts ≥1 with NF1 who have symptomatic, inoperable PNs ascopost.com/news/september… #FDAApproval #Neurofibromatosis #PedsOnc

New FDA approval: Subcutaneous Keytruda Qlex now available for solid tumors. Expect shorter infusion times, fewer central lines, and streamlined care. What to know: bit.ly/4gKEPxV #FDAApproval #OncologyNews #SolidTumors

FDA approves subcutaneous pembrolizumab (Keytruda Qlex) for solid tumors in patients 12+. Same efficacy as IV, faster delivery, less chair time. Learn more: bit.ly/4gKEPxV #FDAApproval #OncologyNews #SolidTumors

🚨 FDA approves Zepzelca (lurbinectedin) in combination with Tecentriq or Tecentriq Hybreza for maintenance treatment of extensive-stage SCLC after first-line therapy clinicaloncology.com/a/fzIBAA/t #SCLC #LungCancer #FDAApproval

From new @US_FDA approvals, to promising clinical trial results, explore @TargetedOnc's weekly roundup of the latest breakthroughs in oncology: bit.ly/42XCyK4 #ClinicalTrials #CancerResearch #FDAApproval #Oncology
Teva’s new bipolar breakthrough: FDA clears UZEDY as the first monthly long-acting risperidone business-news-today.com/tevas-new-bipo… #Teva #UZEDY #FDAApproval #BipolarDisorder #LongActingInjectable #MentalHealth #Psychiatry #PharmaNews #InvestorUpdates #HealthcareInnovation
A big step forward for #HeartFailure care! 💧💉 The new FDA-approved subcutaneous diuretic offers patients a simpler, less invasive option to manage edema potentially reducing hospital visits and improving quality of life. 👏 #CardioTwitter #FDAApproval
UZEDY wins FDA nod for Bipolar I maintenance therapy, boosting Teva and Medincell’s psychiatric drug portfolio business-news-today.com/uzedy-wins-fda… #BipolarIDisorder #FDAApproval #UZEDY #Teva #Medincell #Psychiatry #MentalHealth #LongActingInjectables
A big step forward in #SkinCancer care @US_FDA approves cemiplimab-rwlc for adjuvant treatment of cutaneous SCC. 📊 C-POST trial: Median DFS not reached vs 49.4 mo (placebo); HR 0.32, P < .0001. Stronger protection, longer disease-free survival. 💪 #FDAApproval #Oncology
The @US_FDA has approved cemiplimab-rwlc for the adjuvant treatment of adults with cutaneous squamous cell carcinoma #skincancer #FDAApproval based on findings from the C-POST study - Median DFS not reached vs 49.4 months w/ placebo (HR = 0.32; P < .0001) ascopost.com/news/october-2…

FDA approves Ligand Partner SQ Innovation’s Lasix ONYU for heart failure edema—at-home use marks major shift in care business-news-today.com/fda-approves-l… #LasixONYU #HeartFailure #FDAApproval #LigandPharma #Captisol #BiotechNews #HospitalAtHome #CardiovascularCare #PharmaInnovation
🚨 #FDA approves subcutaneous pembrolizumab (Keytruda Qlex). PK comparability met; ORR 45% SC vs 42% IV in MK-3475A-D77 trial ~1–2 min admin—logistics win, efficacy preserved. #FDAApproval #Oncology #Immunotherapy #CancerResearch #NSCLC #Keytruda #Subcutaneous

🚨 FDA approves Zepzelca (lurbinectedin) in combination with Tecentriq or Tecentriq Hybreza for maintenance treatment of extensive-stage SCLC after first-line therapy clinicaloncology.com/a/fzIBAA/t #SCLC #LungCancer #FDAApproval

The @US_FDA has approved cemiplimab-rwlc for the adjuvant treatment of adults with cutaneous squamous cell carcinoma #skincancer #FDAApproval based on findings from the C-POST study - Median DFS not reached vs 49.4 months w/ placebo (HR = 0.32; P < .0001) ascopost.com/news/october-2…

On August 8, 2025, the FDA grants accelerated approval to zongertinib for non-squamous NSCLC with HER2 TKD activating mutations. Learn more about Beamion LUNG-1 (NCT04886804) 👉 clin.larvol.com/trial-detail/N… #LARVOL #FDAApproval #CancerResearch #Oncology #ClinicalTrials #CancerData…

The FDA has approved a clinical study of an implantable device designed to stabilise blood pressure in people with spinal cord injury. 🔗 Full story: buff.ly/yWVWP5Z #SpinalInjury #NeuroTech #FDAApproval #ARCIM #SCIResearch

The @US_FDA approved selumetinib granules and capsules for pediatric pts ≥1 with NF1 who have symptomatic, inoperable PNs ascopost.com/news/september… #FDAApproval #Neurofibromatosis #PedsOnc

🌟🇺🇸 #FDAApproval Alert🚨 📅 Aug 6, 2025 ➡️ The FDA has granted accelerated approval to dordaviprone (Modeyso) for H3 K27M-mutant diffuse midline glioma in pts ≥1 y/o with progression after prior therapy. This is the first systemic therapy approved for this indication. 🔬 🧪…

New FDA approval: Subcutaneous Keytruda Qlex now available for solid tumors. Expect shorter infusion times, fewer central lines, and streamlined care. What to know: bit.ly/4gKEPxV #FDAApproval #OncologyNews #SolidTumors

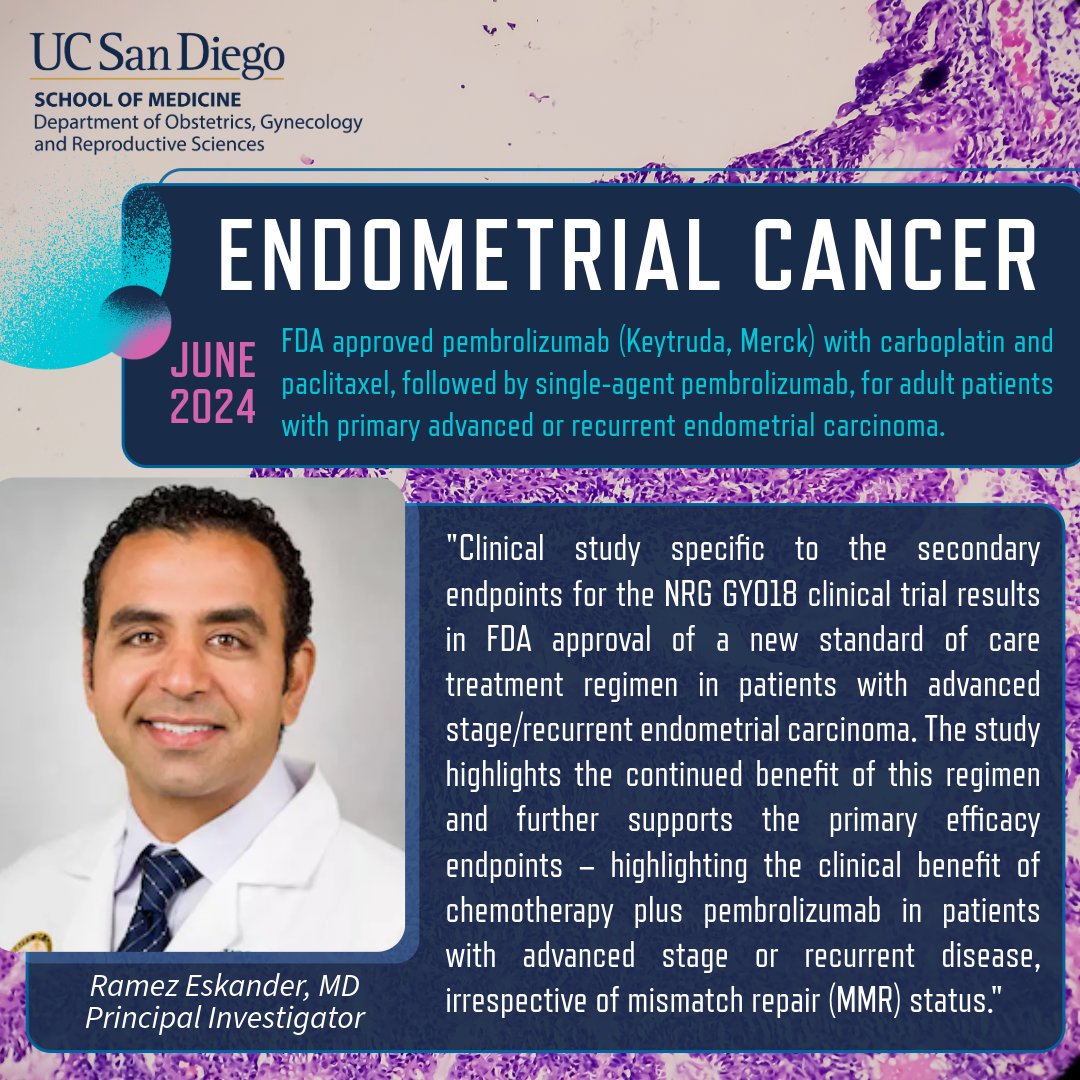
New study by @rne_md @UCSD_ObGyn reinforces prior findings that resulted in #FDAApproval of a new standard of care treatment option in #EndometrialCarcinoma highlighting the clinical benefit of chemotherapy + #pembrolizumab in patients... Discover study ⬇️ go.ucsd.edu/422V83j
Prescribing patterns for cancer therapies rose more after accelerated approval than after regular approval by the FDA, with off-label use remaining uncommon. Study led by: @ravi_b_parikh. Read more → ascopost.com/news/july-2025… #FDAApproval #CancerDrugs

The @US_FDA has approved lurbinectedin + atezolizumab OR atezolizumab + hyaluronidase-tqjs as maintenance for adults with ES-SCLC without progression on first-line atezolizumab ± hyaluronidase-tqjs, carboplatin, etoposide #FDAApproval #SCLC ascopost.com/news/october-2…

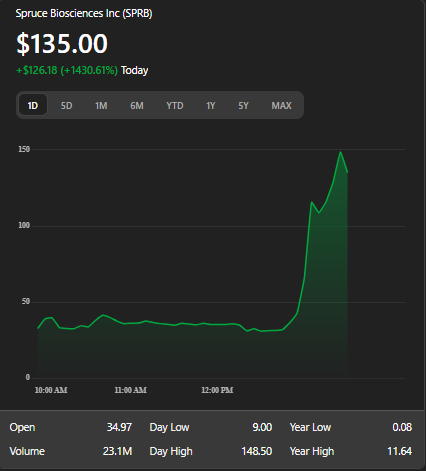
The stock opened at $34.97, reached a high of $148.50, and is currently trading at $135.00. With a market cap of approximately $60.3 million, the surge reflects strong investor optimism #SPRB #BiotechBreakthrough #FDAApproval #StockSurge #Investing #PharmaNews #BiotechStocks
Florida’s rethinking school lunches 🍎 New UTI antibiotic 💊 Alzheimer’s blood test 🧠 Microplastics in gum 😬 And yep, another pig kidney transplant 🐷 Full breakdown in our latest episode 🎧 #MedicalNews #FDAApproval #SchoolNutrition #Alzheimers #UTI #PublicHealth

Biocon Biologics Gets US FDA Approval for Insulin Aspart 'Kirsty' #BioconBiologics #Kirsty #FDAApproval #InsulinBiosimilar #DiabetesCare #AffordableInsulin #Biosimilars #NovoLogAlternative

The @US_FDA approved estrogen receptor antagonist imlunestrant for pts with ER+/HER2– ESR1-mutant advanced/metastatic breast cancer with disease progression after ≥1 endocrine therapy ➡️Companion diagnostic also approved ascopost.com/news/september… #BCSM #BreastCancer #FDAApproval

FDA grants accelerated approval to dordaviprone (Modeyso) for H3 K27M–mutant diffuse midline glioma. 22% response rate; median response duration 10.3 months. Study by Arrillaga-Romany et al. 🔗 ascopost.com/news/august-20… #NeuroOncology #FDAApproval

The @US_FDA approved pembrolizumab and berahyaluronidase alfa-pmph (Keytruda Qlex) for subcutaneous (#SQ) injection for all pembrolizumab intravenous (#IV) adult and pediatric solid tumor indications ascopost.com/news/september… #FDAApproval

FDA approves subcutaneous pembrolizumab (Keytruda Qlex) for solid tumors in patients 12+. Same efficacy as IV, faster delivery, less chair time. Learn more: bit.ly/4gKEPxV #FDAApproval #OncologyNews #SolidTumors

‼️ Big news in the world of breast reconstruction: The FDA has approved Mentor MemoryGel Enhance implants, offering sizes from 930 cc to 1445 cc ‼️ Here’s what this means for breast cancer survivors. 🧵👇 #BreastReconstruction #FDAApproval

FDA Approves First Pig Kidney Transplant Trials, Offering Hope for Organ Shortage. #KidneyTransplant #OrganShortage #FDAApproval #MedicalInnovation #Xenotransplantation

Something went wrong.
Something went wrong.
United States Trends
- 1. Auburn 46.2K posts
- 2. At GiveRep N/A
- 3. Brewers 65.7K posts
- 4. Cubs 56.8K posts
- 5. #SEVENTEEN_NEW_IN_TACOMA 33.6K posts
- 6. Georgia 68.6K posts
- 7. Gilligan's Island 4,863 posts
- 8. #byucpl N/A
- 9. Utah 25.5K posts
- 10. MACROHARD 4,674 posts
- 11. Arizona 42.1K posts
- 12. Kirby 24.3K posts
- 13. Wordle 1,576 X N/A
- 14. #AcexRedbull 4,304 posts
- 15. Michigan 63.3K posts
- 16. #SVT_TOUR_NEW_ 25.3K posts
- 17. Boots 51.4K posts
- 18. #Toonami 2,997 posts
- 19. mingyu 92.3K posts
- 20. Hugh Freeze 3,283 posts



























